Mechanisms of polarized membrane trafficking in neurons -- focusing in on endosomes
- PMID: 21762782
- PMCID: PMC3205304
- DOI: 10.1016/j.mcn.2011.06.013
Mechanisms of polarized membrane trafficking in neurons -- focusing in on endosomes
Abstract
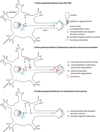
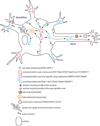
Neurons are polarized cells that have a complex and unique morphology: long processes (axons and dendrites) extending far from the cell body. In addition, the somatodendritic and axonal domains are further divided into specific subdomains, such as synapses (pre- and postsynaptic specializations), proximal and distal dendrites, axon initial segments, nodes of Ranvier, and axon growth cones. The striking asymmetry and complexity of neuronal cells are necessary for their function in receiving, processing and transferring electrical signals, with each domain playing a precise function in these processes. In order to establish and maintain distinct neuronal domains, mechanisms must exist for protein delivery to specific neuronal compartments, such that each compartment has the correct functional molecular composition. How polarized membrane domains are established and maintained is a long-standing question. Transmembrane proteins, such as receptors and adhesion molecules, can be transported to their proper membrane domains by several pathways. The biosynthetic secretory system delivers newly synthesized transmembrane proteins from the ER via the Golgi and trans-Golgi-network (TGN) to the plasma membrane. In addition, the endosomal system is critically involved in many instances in ensuring proper (re)targeting of membrane components because it can internalize and degrade mislocalized proteins, or recycle proteins from one domain to another. The endosomal system is thus crucial for establishing and maintaining neuronal polarity. In this review, we focus mainly on the intracellular compartments that serve as sorting stations for polarized transport, with particular emphasis on the emerging roles of endosomes.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- Alberi S, Boda B, Steiner P, Nikonenko I, Hirling H, Muller D. The endosomal protein NEEP21 regulates AMPA receptor-mediated synaptic transmission and plasticity in the hippocampus. Mol Cell Neurosci. 2005;29:313–319. - PubMed
-
- Andreassi C, Riccio A. To localize or not to localize: mRNA fate is in 3'UTR ends. Trends Cell Biol. 2009;19:465–474. - PubMed
-
- Arimura N, Kimura T, Nakamuta S, Taya S, Funahashi Y, Hattori A, Shimada A, Menager C, Kawabata S, Fujii K, Iwamatsu A, Segal RA, Fukuda M, Kaibuchi K. Anterograde transport of TrkB in axons is mediated by direct interaction with Slp1 and Rab27. Dev Cell. 2009;16:675–686. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

