Regression of allograft airway fibrosis: the role of MMP-dependent tissue remodeling in obliterative bronchiolitis after lung transplantation
- PMID: 21763265
- PMCID: PMC3157248
- DOI: 10.1016/j.ajpath.2011.05.032
Regression of allograft airway fibrosis: the role of MMP-dependent tissue remodeling in obliterative bronchiolitis after lung transplantation
Abstract
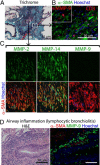
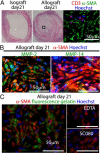
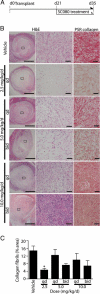
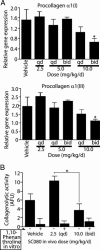
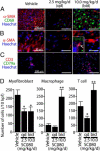
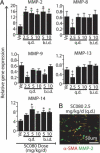
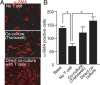
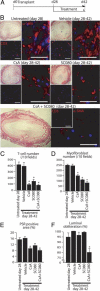
Obliterative bronchiolitis after lung transplantation is a chronic inflammatory and fibrotic condition of small airways. The fibrosis associated with obliterative bronchiolitis might be reversible. Matrix metalloproteinases (MMPs) participate in inflammation and tissue remodeling. MMP-2 localized to myofibroblasts in post-transplant human obliterative bronchiolitis lesions and to allograft fibrosis in a rat intrapulmonary tracheal transplant model. Small numbers of infiltrating T cells were also observed within the fibrosis. To modulate inflammation and tissue remodeling, the broad-spectrum MMP inhibitor SC080 was administered after the allograft was obliterated, starting at post-transplant day 21. The allograft lumen remained obliterated after treatment. Only low-dose (2.5 mg/kg per day) SC080 significantly reduced collagen deposition, reduced the number of myofibroblasts and the infiltration of T cells in association with increased collagenolytic activity, increased MMP-2 gene expression, and decreased MMP-8, MMP-9, and MMP-13 gene expression. In in vitro experiments using cultured myofibroblasts, a relatively low concentration of SC080 increased MMP-2 activity and degradation of type I collagen. Moreover, coculture with T cells facilitated persistence of myofibroblasts, suggesting a role for T-cell infiltration in myofibroblast persistence in fibrosis. By combining low-dose SC080 with cyclosporine in vivo at post-transplant day 28, partial reversal of obliterative fibrosis was observed at day 42. Thus, modulating MMP activity might reverse established allograft airway fibrosis by regulating inflammation and tissue remodeling.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
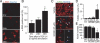

References
-
- Stewart S., Fishbein M.C., Snell G.I., Berry G.J., Boehler A., Burke M.M., Glanville A., Gould F.K., Magro C., Marboe C.C., McNeil K.D., Reed E.F., Reinsmoen N.L., Scott J.P., Studer S.M., Tazelaar H.D., Wallwork J.L., Westall G., Zamora M.R., Zeevi A., Yousem S.A. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. - PubMed
-
- Nicod L.P. Mechanisms of airway obliteration after lung transplantation. Proc Am Thorac Soc. 2006;3:444–449. - PubMed
-
- Boehler A., Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J. 2003;22:1007–1018. - PubMed
-
- Kumar V., Abbas A.K., Fausto N. Robbins & Cotran Pathologic Basis of Disease. ed 7. Elsevier Saunders; Philadelphia: 2005. Tissue renewal and repair: regeneration, healing and fibrosis; pp. 87–118.
-
- Iimuro Y., Nishio T., Morimoto T., Nitta T., Stefanovic B., Choi S.K., Brenner D.A., Yamaoka Y. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

