Microfluidic devices for modeling cell-cell and particle-cell interactions in the microvasculature
- PMID: 21763328
- PMCID: PMC3215799
- DOI: 10.1016/j.mvr.2011.06.013
Microfluidic devices for modeling cell-cell and particle-cell interactions in the microvasculature
Abstract
Cell-fluid and cell-cell interactions are critical components of many physiological and pathological conditions in the microvasculature. Similarly, particle-cell interactions play an important role in targeted delivery of therapeutics to tissue. Development of in vitro fluidic devices to mimic these microcirculatory processes has been a critical step forward in our understanding of the inflammatory process, developing of nano-particulate drug carriers, and developing realistic in vitro models of the microvasculature and its surrounding tissue. However, widely used parallel plate flow based devices and assays have a number of important limitations for studying the physiological conditions in vivo. In addition, these devices are resource hungry and time consuming for performing various assays. Recently developed, more realistic, microfluidic based devices have been able to overcome many of these limitations. In this review, an overview of the fluidic devices and their use in studying the effects of shear forces on cell-cell and cell-particle interactions is presented. In addition, use of mathematical models and computational fluid dynamics (CFD) based models for interpreting the complex flow patterns in the microvasculature is highlighted. Finally, the potential of 3D microfluidic devices and imaging for better representing in vivo conditions under which cell-cell and cell-particle interactions take place is discussed.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

Schematic of the leukocyte adhesion process. Leukocytes roll on the endothelium followed by adhesion, spreading and subsequent migration into the tissue space
Example of a common biophysical process during cell Cells/particles adhere when the adhesive forces are equal or greater than the hydrodynamic forces acting on the cell. Although the illustration shows the hydrodynamic force parallel to the surface, the realized force may have a new orientation following rolling the adhesive forces formed between the ligand and receptors will not necessarily be normal to the surface at all times during the adhesion process.
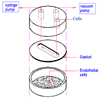

µ-Slide (Ibidi LLC) for adhesion assays
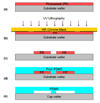
Fluxion Biosciences Well plate based high-throughput assay for adhesion assays






References
-
- Abdelgawad M, Wu C, Chien WY, Geddie WR, Jewett MA, Sun Y. A fast and simple method to fabricate circular microchannels in polydimethylsiloxane (PDMS) Lab Chip. 7. 2011;11(3):545–551. - PubMed
-
- Aigner S, Ramos CL, Hafezi-Moghadam A, Lawrence MB, Friederichs J, Altevogt P, Ley K. CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB J. 1998;12(12):1241–1251. - PubMed
-
- Alves CS, Burdick MM, Thomas SN, Pawar P, Konstantopoulos K. The dual role of CD44 as a functional P-selectin ligand and fibrin receptor in colon carcinoma cell adhesion. Am J Physiol Cell Physiol. 2008;294(4):C907–C916. - PubMed
-
- Anderson JR, Chiu DT, Jackman RJ, Cherniavskaya O, McDonald JC, Wu H, Whitesides SH, Whitesides GM. Fabrication of topologically complex three-dimensional microfluidic systems in PDMS by rapid prototyping. Anal Chem 15. 2000;72(14):3158–3164. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

