Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation
- PMID: 21763616
- PMCID: PMC6894370
- DOI: 10.1016/j.devcel.2011.06.018
Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation
Abstract
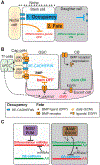
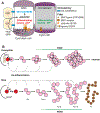
The past decade of research on Drosophila stem cells and niches has provided key insights. Fly stem cells do not occupy a special "state" based on novel "stem cell genes" but resemble transiently arrested tissue progenitors. Moreover, individual stem cells and downstream progenitors are highly dynamic and dispensable, not tissue bulwarks. Niches, rather than fixed cell lineages, ensure tissue health by holding stem cells and repressing cell differentiation inside, but not outside. We review the five best-understood adult Drosophila stem cells and argue that the fundamental biology of stem cells and niches is conserved between Drosophila and mice.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing interests statement
The authors declare that they have no competing financial interests.
Figures


References
-
- Asaoka-Taguchi M, Yamada M, Nakamura A, Hanyu K, and Kobayashi S (1999). Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nat Cell Biol. 1, 431–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

