Orally administered prion protein is incorporated by m cells and spreads into lymphoid tissues with macrophages in prion protein knockout mice
- PMID: 21763679
- PMCID: PMC3157171
- DOI: 10.1016/j.ajpath.2011.05.058
Orally administered prion protein is incorporated by m cells and spreads into lymphoid tissues with macrophages in prion protein knockout mice
Abstract
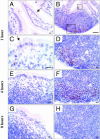
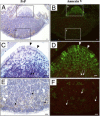
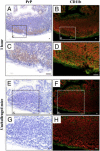
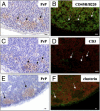
Transmissible spongiform encephalopathies are fatal neurodegenerative diseases. Infection by the oral route is assumed to be important, although its pathogenesis is not understood. Using prion protein (PrP) knockout mice, we investigated the sequence of events during the invasion of orally administered PrPs through the intestinal mucosa and the spread into lymphoid tissues and the peripheral nervous system. Orally administered PrPs were incorporated by intestinal epitheliocytes in the follicle-associated epithelium and villi within 1 hour. PrP-positive cells accumulated in the subfollicle region of Peyer's patches a few hours thereafter. PrP-positive cells spread toward the mesenteric lymph nodes and spleen after the accumulation of PrPs in the Peyer's patches. The number of PrP molecules in the mesenteric lymph nodes and spleen peaked at 2 days and 6 days after inoculation, respectively. The epitheliocytes in the follicle-associated epithelium incorporating PrPs were annexin V-positive microfold cells and PrP-positive cells in Peyer's patches and spleen were CD11b-positive and CD14-positive macrophages. Additionally, PrP-positive cells in Peyer's patches and spleen were detected in the vicinity of peripheral nerve fibers in the early stages of infection. These results indicate that orally delivered PrPs were incorporated by microfold cells promptly after challenge and that macrophages might act as a transporter of incorporated PrPs from the Peyer's patches to other lymphoid tissues and the peripheral nervous system.
Copyright © 2011. Published by Elsevier Inc.
Figures



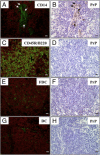
References
-
- Aguzzi A. Prion diseases of humans and farm animals: epidemiology, genetics and pathogenesis. J Neurochem. 2006;97:1726–1739. - PubMed
-
- Masters C.L., Richardson E.P., Jr Subacute spongiform encephalopathy (Creutzfeldt-Jakob Disease): The nature and progression of spongiform change. Brain. 1978;101:333–344. - PubMed
-
- van Everbroeck B., Dobbeleir I., De Waele M., De Leenheir E., Lubke U., Martin J.J., Cras P. Extracellular protein deposition correlates with glial activation and oxidative stress in Creutzfeldt-Jakob and Alzheimer's disease. Acta Neuropathol. 2004;108:194–200. - PubMed
-
- Bolton D.C., McKinley M.P., Prusiner S.B. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

