Patient-specific modeling of dyssynchronous heart failure: a case study
- PMID: 21763714
- PMCID: PMC3190065
- DOI: 10.1016/j.pbiomolbio.2011.06.014
Patient-specific modeling of dyssynchronous heart failure: a case study
Abstract
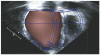
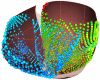
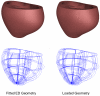
The development and clinical use of patient-specific models of the heart is now a feasible goal. Models have the potential to aid in diagnosis and support decision-making in clinical cardiology. Several groups are now working on developing multi-scale models of the heart for understanding therapeutic mechanisms and better predicting clinical outcomes of interventions such as cardiac resynchronization therapy. Here we describe the methodology for generating a patient-specific model of the failing heart with a myocardial infarct and left ventricular bundle branch block. We discuss some of the remaining challenges in developing reliable patient-specific models of cardiac electromechanical activity, and identify some of the main areas for focusing future research efforts. Key challenges include: efficiently generating accurate patient-specific geometric meshes and mapping regional myofiber architecture to them; modeling electrical activation patterns based on cellular alterations in human heart failure, and estimating regional tissue conductivities based on clinically available electrocardiographic recordings; estimating unloaded ventricular reference geometry and material properties for biomechanical simulations; and parameterizing systemic models of circulatory dynamics from available hemodynamic measurements.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
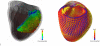






References
-
- Alastrué V, Martínez MA, Doblaré M. Modelling adaptative volumetric finite growth in patient-specific residually stressed arteries. Journal of Biomechanics. 2008;41:1773–1781. - PubMed
-
- Arsigny V, Fillard P, Pennec X, Ayache N. Log-Euclidian metrics for fast and simple calculus on diffusion tensors. Magnetic Reonance in Medicine. 2006;56:411–421. - PubMed
-
- Arts T, Delhaas T, Bovendeerd P, Verbeek X, Prinzen FW. Adaptation to mechanical load determines shape and properties of heart and circulation: the CircAdapt model. American Journal of Physiology-Heart and Circulatory Physiology. 2005;288:H1943–H1954. - PubMed
-
- Arts T, Lumens J, Kroon W, Donker D, Prinzen F, Delhaas T. Patient-Specific Modeling of Cardiovascular Dynamics with a Major Role for Adaptation. In: Kerckhoffs RCP, editor. Patient-Specific Modeling of the Cardiovascular System. Springer; New York: 2010. pp. 21–41.
-
- Boemser P, Ranke O. Ueber die messung des schlagvolume des herzens auf unglutigem. Weg. Zeitung fuer Biologie. 1930;90:467–507.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

