The discovery and development of inhibitors of fatty acid amide hydrolase (FAAH)
- PMID: 21764305
- PMCID: PMC3146581
- DOI: 10.1016/j.bmcl.2011.06.096
The discovery and development of inhibitors of fatty acid amide hydrolase (FAAH)
Abstract
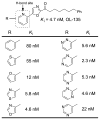
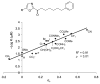
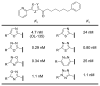
A summary of the discovery and advancement of inhibitors of fatty acid amide hydrolase (FAAH) is presented.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
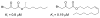



















Similar articles
-
Discovery of boronic acids as novel and potent inhibitors of fatty acid amide hydrolase.J Med Chem. 2008 Nov 27;51(22):7057-60. doi: 10.1021/jm801051t. J Med Chem. 2008. PMID: 18983140
-
Discovery and development of fatty acid amide hydrolase (FAAH) inhibitors.J Med Chem. 2008 Dec 11;51(23):7327-43. doi: 10.1021/jm800311k. J Med Chem. 2008. PMID: 18983142 Review. No abstract available.
-
Mining biologically-active molecules for inhibitors of fatty acid amide hydrolase (FAAH): identification of phenmedipham and amperozide as FAAH inhibitors.Bioorg Med Chem Lett. 2009 Dec 1;19(23):6793-6. doi: 10.1016/j.bmcl.2009.09.086. Epub 2009 Sep 30. Bioorg Med Chem Lett. 2009. PMID: 19850474
-
Fatty acid amide hydrolase inhibitors. 3: tetra-substituted azetidine ureas with in vivo activity.Bioorg Med Chem Lett. 2012 Jan 15;22(2):901-6. doi: 10.1016/j.bmcl.2011.12.032. Epub 2011 Dec 13. Bioorg Med Chem Lett. 2012. PMID: 22209458
-
Application of computational methods to the design of fatty acid amide hydrolase (FAAH) inhibitors based on a carbamic template structure.Adv Protein Chem Struct Biol. 2011;85:1-26. doi: 10.1016/B978-0-12-386485-7.00001-6. Adv Protein Chem Struct Biol. 2011. PMID: 21920320 Review.
Cited by
-
Piperidinyl thiazole isoxazolines: A new series of highly potent, slowly reversible FAAH inhibitors with analgesic properties.Bioorg Med Chem Lett. 2016 Jun 15;26(12):2965-2973. doi: 10.1016/j.bmcl.2016.02.061. Epub 2016 Feb 22. Bioorg Med Chem Lett. 2016. PMID: 27130358 Free PMC article.
-
A binding site for nonsteroidal anti-inflammatory drugs in fatty acid amide hydrolase.J Am Chem Soc. 2013 Jan 9;135(1):22-5. doi: 10.1021/ja308733u. Epub 2012 Dec 21. J Am Chem Soc. 2013. PMID: 23240907 Free PMC article.
-
Inhibition of FAAH, TRPV1, and COX2 by NSAID-serotonin conjugates.Bioorg Med Chem Lett. 2014 Dec 15;24(24):5695-5698. doi: 10.1016/j.bmcl.2014.10.064. Epub 2014 Oct 28. Bioorg Med Chem Lett. 2014. PMID: 25467164 Free PMC article.
-
Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors.ACS Chem Neurosci. 2013 Sep 18;4(9):1322-32. doi: 10.1021/cn400116z. Epub 2013 Jun 17. ACS Chem Neurosci. 2013. PMID: 23731016 Free PMC article.
-
Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain.Nat Rev Drug Discov. 2012 Apr;11(4):292-310. doi: 10.1038/nrd3673. Nat Rev Drug Discov. 2012. PMID: 22460123 Review.
References
-
-
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946.Review: Martin BR, Mechoulam R, Razdan RK. Life Sci. 1999;65:573.
-
-
-
Cravatt BF, Lerner RA, Boger DL. J Am Chem Soc. 1996;118:580.Cravatt BF, Prospero–Garcia O, Suizdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA. Science. 1995;268:1506.Lerner RA, Siuzdak G, Prospero Garcia O, Henriksen SJ, Boger DL, Cravatt BF. Proc Natl Acad Sci USA. 1994;91:9505.Review: Boger DL, Henriksen SJ, Cravatt BF. Curr Pharm Des. 1998;4:303.
-
-
-
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Nature. 1996;384:83.Giang DK, Cravatt BF. Proc Natl Acad Sci USA. 1997;94:2238.Reviews: Patricelli MP, Cravatt BF. Vit Hormones. 2001;62:95.McKinney MK, Cravatt BF. Ann Rev Biochem. 2005;74:411.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

