(124)I-huA33 antibody PET of colorectal cancer
- PMID: 21764796
- PMCID: PMC3394182
- DOI: 10.2967/jnumed.110.086165
(124)I-huA33 antibody PET of colorectal cancer
Abstract
Humanized A33 (huA33) is a promising monoclonal antibody that recognizes A33 antigen, which is present in more than 95% of colorectal cancers and in normal bowel. In this study, we took advantage of quantitative PET to evaluate (124)I huA33 targeting, biodistribution, and safety in patients with colorectal cancer. We also determined the biodistribution of (124)I-huA33 when a large dose of human intravenous IgG (IVIG) was administered to manipulate the Fc receptor or when (124)I-huA33 was given via hepatic arterial infusion (HAI).
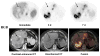
Methods: We studied 25 patients with primary or metastatic colorectal cancer; 19 patients had surgical exploration or resection. Patients received a median of 343 MBq (44.4-396 MBq) and 10 mg of (124)I-huA33. Nineteen patients received the antibody intravenously and 6 patients via HAI, and 5 patients also received IVIG.
Results: Ten of 12 primary tumors were visualized in 11 patients. The median concentration in primary colon tumors was 0.016% injected dose per gram, compared with 0.004% in normal colon. The PET-based median ratio of hepatic tumor uptake to normal-liver uptake was 3.9 (range, 1.8-22.2). Quantitation using PET, compared with well counting of serum and tissue, showed little difference. Prominent uptake in bowel hindered tumor identification in some patients. Pharmacokinetics showed that patients receiving IVIG had a significantly shorter serum half-time (41.6 ± 14.0 h) than those without (65.2 ± 9.8 h). There were no differences in clearance rates among the intravenous group, IVIG group, and HAI group, nor was there any difference in serum area under the curve, maximum serum concentration, or volume of distribution. Weak titers of human-antihuman antibodies were observed in 6 of 25 patients. No acute side effects or significant toxicities were associated with huA33.
Conclusion: Good localization of (124)I-huA33 in colorectal cancer with no significant toxicity has been observed. PET-derived (124)I concentrations agreed well with those obtained by well counting of surgically resected tissue and blood, confirming the quantitative accuracy of (124)I-huA33 PET. The HAI route had no advantage over the intravenous route. No clinically significant changes in blood clearance were induced by IVIG.
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures


Comment in
-
Immuno-PET of cancer: a revival of antibody imaging.J Nucl Med. 2011 Aug;52(8):1171-2. doi: 10.2967/jnumed.111.089771. Epub 2011 Jul 15. J Nucl Med. 2011. PMID: 21764784 No abstract available.
References
-
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. - PubMed
-
- Divgi CR, Pandit-Taskar N, Jungbluth AA, et al. Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol. 2007;8:304–310. - PubMed
-
- Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with meta-static breast cancer. Clin Pharmacol Ther. 2010;87:586–592. - PubMed
-
- Sakamoto J, Kojima H, Kato J, Hamashima H, Suzuki H. Organ-specific expression of the intestinal epithelium-related antigen A33, a cell surface target for antibody-based imaging and treatment in gastrointestinal cancer. Cancer Chemother Pharmacol. 2000;46:S27–S32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
