The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections
- PMID: 21764818
- PMCID: PMC3155707
- DOI: 10.1093/brain/awr161
The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections
Abstract
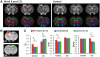
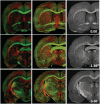
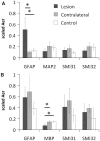
Diffusion tensor imaging is highly sensitive to the microstructural integrity of the brain and has uncovered significant abnormalities following traumatic brain injury not appreciated through other methods. It is hoped that this increased sensitivity will aid in the detection and prognostication in patients with traumatic injury. However, the pathological substrates of such changes are poorly understood. Specifically, decreases in fractional anisotropy derived from diffusion tensor imaging are consistent with axonal injury, myelin injury or both in white matter fibres. In contrast, in both humans and animal models, increases in fractional anisotropy have been suggested to reflect axonal regeneration and plasticity, but the direct histological evidence for such changes remains tenuous. We developed a method to quantify the anisotropy of stained histological sections using Fourier analysis, and applied the method to a rat controlled cortical impact model to identify the specific pathological features that give rise to the diffusion tensor imaging changes in subacute to chronic traumatic brain injury. A multiple linear regression was performed to relate the histological measurements to the measured diffusion tensor changes. The results show that anisotropy was significantly increased (P < 0.001) in the perilesioned cortex following injury. Cortical anisotropy was independently associated (standardized β = 0.62, P = 0.04) with the coherent organization of reactive astrocytes (i.e. gliosis) and was not attributed to axons. By comparison, a decrease in white matter anisotropy (P < 0.001) was significantly related to demyelination (β = 0.75, P = 0.0015) and to a lesser extent, axonal degeneration (β = -0.48, P = 0.043). Gliosis within the lesioned cortex also influenced diffusion tensor tractography, highlighting the fact that spurious tracts in the injured brain may not necessarily reflect continuous axons and may instead depict glial scarring. The current study demonstrates a novel method to relate pathology to diffusion tensor imaging findings, elucidates the underlying mechanisms of anisotropy changes following traumatic brain injury and significantly impacts the clinical interpretation of diffusion tensor imaging findings in the injured brain.
Figures




References
-
- Armitage PA, Bastin ME. Selecting an appropriate anisotropy index for displaying diffusion tensor imaging data with improved contrast and sensitivity. Magn Reson Med. 2000;44:117–21. - PubMed
-
- Armitage PA, Bastin ME, Marshall I, Wardlaw JM, Cannon J. Diffusion anisotropy measurements in ischaemic stroke of the human brain. MAGMA. 1998;6:28–36. - PubMed
-
- Axer M, Amunts K, Grässel D, Palm C, Dammers J, Axer H, et al. A novel approach to the human connectome: ultra-high resolution mapping of fiber tracts in the brain. NeuroImage. 2011;54:1091–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

