Systematic analysis of diguanylate cyclases that promote biofilm formation by Pseudomonas fluorescens Pf0-1
- PMID: 21764921
- PMCID: PMC3165641
- DOI: 10.1128/JB.05483-11
Systematic analysis of diguanylate cyclases that promote biofilm formation by Pseudomonas fluorescens Pf0-1
Abstract
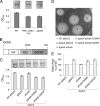
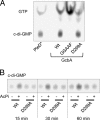
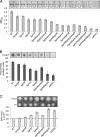
Cyclic di-GMP (c-di-GMP) is a broadly conserved, intracellular second-messenger molecule that regulates biofilm formation by many bacteria. The synthesis of c-di-GMP is catalyzed by diguanylate cyclases (DGCs) containing the GGDEF domain, while its degradation is achieved through the phosphodiesterase activities of EAL and HD-GYP domains. c-di-GMP controls biofilm formation by Pseudomonas fluorescens Pf0-1 by promoting the cell surface localization of a large adhesive protein, LapA. LapA localization is regulated posttranslationally by a c-di-GMP effector system consisting of LapD and LapG, which senses cytoplasmic c-di-GMP and modifies the LapA protein in the outer membrane. Despite the apparent requirement for c-di-GMP for biofilm formation by P. fluorescens Pf0-1, no DGCs from this strain have been characterized to date. In this study, we undertook a systematic mutagenesis of 30 predicted DGCs and found that mutations in just 4 cause reductions in biofilm formation by P. fluorescens Pf0-1 under the conditions tested. These DGCs were characterized genetically and biochemically to corroborate the hypothesis that they function to produce c-di-GMP in vivo. The effects of DGC gene mutations on phenotypes associated with biofilm formation were analyzed. One DGC preferentially affects LapA localization, another DGC mainly controls swimming motility, while a third DGC affects both LapA and motility. Our data support the conclusion that different c-di-GMP-regulated outputs can be specifically controlled by distinct DGCs.
Copyright © 2011, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Christen M., Christen B., Folcher M., Schauerte A., Jenal U. 2005. Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J. Biol. Chem. 280:30829–30837 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

