FLT3 inhibition as therapy in acute myeloid leukemia: a record of trials and tribulations
- PMID: 21765192
- PMCID: PMC3228152
- DOI: 10.1634/theoncologist.2011-0084
FLT3 inhibition as therapy in acute myeloid leukemia: a record of trials and tribulations
Abstract
Acute myeloid leukemia (AML) is a hematologic malignancy with a poor prognosis. Approximately one quarter of the patients with AML also carry an internal tandem duplication (ITD) mutation in the gene encoding FMS-like tyrosine kinase 3 (FLT3), which has a significantly deleterious impact on prognosis. The ITD mutation renders FLT3 constitutively active and leads to uncontrolled proliferation of the leukemic blast. Over the course of the last decade, a variety of compounds have been developed in preclinical and clinical studies as potent inhibitors of FLT3. Many of the earlier agents under investigation, such as lestaurtinib, midostaurin, and sunitinib, were initially developed as inhibitors of other tyrosine kinases and as targeted therapies in a variety of malignancies. These compounds have been demonstrated to have some efficacy in clinical trials of AML, mainly manifesting as transient decreases in circulating blasts correlating with effective in vivo suppression of the FLT3 target. Nevertheless, the cumbersome pharmacokinetics of some compounds and the suboptimal specificity and potency of others have limited their therapeutic efficacy. In the last few years, newer, more potent and specific agents have been under investigation, with the leading example being AC220. This agent has shown significant promise in early phases of clinical investigation, and is currently in more advanced clinical trials. Hope remains that FLT3 inhibition will be become an effective therapeutic adjunct to our current treatment approach to AML.
Conflict of interest statement
Section Editor
Section Editor
Reviewers “A” and “B” disclose no financial relationships.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. On the basis of disclosed information, all conflicts of interest have been resolved.
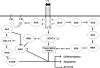
Figures
Similar articles
-
FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML.Blood. 2010 Feb 18;115(7):1425-32. doi: 10.1182/blood-2009-09-242859. Epub 2009 Dec 10. Blood. 2010. PMID: 20007803 Free PMC article.
-
The secondary FLT3-ITD F691L mutation induces resistance to AC220 in FLT3-ITD+ AML but retains in vitro sensitivity to PKC412 and Sunitinib.Leukemia. 2013 Jun;27(6):1416-8. doi: 10.1038/leu.2013.14. Epub 2013 Jan 16. Leukemia. 2013. PMID: 23392356 No abstract available.
-
FLT3 inhibitors in acute myeloid leukemia: ten frequently asked questions.Leukemia. 2020 Mar;34(3):682-696. doi: 10.1038/s41375-019-0694-3. Epub 2020 Jan 9. Leukemia. 2020. PMID: 31919472 Review.
-
The role of quizartinib in the treatment of acute myeloid leukemia.Expert Opin Investig Drugs. 2013 Dec;22(12):1659-69. doi: 10.1517/13543784.2013.842973. Epub 2013 Sep 26. Expert Opin Investig Drugs. 2013. PMID: 24070241 Review.
-
FLT3 inhibitors in the treatment of acute myeloid leukemia: current status and future perspectives.Minerva Med. 2020 Oct;111(5):427-442. doi: 10.23736/S0026-4806.20.06989-X. Epub 2020 Sep 21. Minerva Med. 2020. PMID: 32955823 Review.
Cited by
-
Using functional genomics to overcome therapeutic resistance in hematological malignancies.Immunol Res. 2013 Mar;55(1-3):100-15. doi: 10.1007/s12026-012-8353-z. Immunol Res. 2013. PMID: 22941562 Free PMC article. Review.
-
Preclinical antitumor activity of ST7612AA1: a new oral thiol-based histone deacetylase (HDAC) inhibitor.Oncotarget. 2015 Mar 20;6(8):5735-48. doi: 10.18632/oncotarget.3240. Oncotarget. 2015. PMID: 25671299 Free PMC article.
-
Gene mutations and molecularly targeted therapies in acute myeloid leukemia.Am J Blood Res. 2013;3(1):29-51. Epub 2013 Jan 17. Am J Blood Res. 2013. PMID: 23358589 Free PMC article.
-
Preclinical antitumor activity of SST0116CL1: a novel heat shock protein 90 inhibitor.Int J Oncol. 2014 Oct;45(4):1421-9. doi: 10.3892/ijo.2014.2575. Epub 2014 Aug 1. Int J Oncol. 2014. PMID: 25096516 Free PMC article.
-
Synergistic cytotoxicity of sorafenib with busulfan and nucleoside analogs in human FMS-like tyrosine kinase 3 internal tandem duplications-positive acute myeloid leukemia cells.Biol Blood Marrow Transplant. 2014 Nov;20(11):1687-95. doi: 10.1016/j.bbmt.2014.08.003. Epub 2014 Aug 9. Biol Blood Marrow Transplant. 2014. PMID: 25111583 Free PMC article.
References
-
- Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–2784. - PubMed
-
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. - PubMed
-
- Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–1752. - PubMed
-
- Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. - PubMed
-
- Knapper S, Mills KI, Gilkes AF, et al. The effects of lestaurtinib (CEP701) and PKC412 on primary AML blasts: The induction of cytotoxicity varies with dependence on FLT3 signaling in both FLT3-mutated and wild-type cases. Blood. 2006;108:3494–3503. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

