Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway
- PMID: 21765214
- PMCID: PMC3148723
- DOI: 10.1172/JCI43124
Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway
Abstract
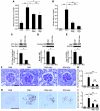
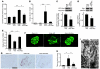
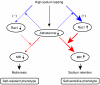
Hypertension is a leading contributor to cardiovascular mortality worldwide. Despite this, its underlying mechanism(s) and the role of excess salt in cardiorenal dysfunction are unclear. Previously, we have identified cross-talk between mineralocorticoid receptor (MR), a nuclear transcription factor regulated by the steroid aldosterone, and the small GTPase Rac1, which is implicated in proteinuric kidney disease. We here show that high-salt loading activates Rac1 in the kidneys in rodent models of salt-sensitive hypertension, leading to blood pressure elevation and renal injury via an MR-dependent pathway. We found that a high-salt diet caused renal Rac1 upregulation in salt-sensitive Dahl (Dahl-S) rats and downregulation in salt-insensitive Dahl (Dahl-R) rats. Despite a reduction of serum aldosterone levels, salt-loaded Dahl-S rats showed increased MR signaling in the kidneys, and Rac1 inhibition prevented hypertension and renal damage with MR repression. We further demonstrated in aldosterone-infused rats as well as adrenalectomized Dahl-S rats with aldosterone supplementation that salt-induced Rac1 and aldosterone acted interdependently to cause MR overactivity and hypertension. Finally, we confirmed the key role of Rac1 in modulating salt susceptibility in mice lacking Rho GDP-dissociation inhibitor α. Therefore, our data identify Rac1 as a determinant of salt sensitivity and provide insights into the mechanism of salt-induced hypertension and kidney injury.
Figures

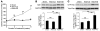
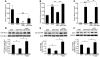

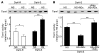
References
-
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

