Induction of cellular antioxidant defense by amifostine improves ventilator-induced lung injury
- PMID: 21765345
- PMCID: PMC3657468
- DOI: 10.1097/CCM.0b013e3182284a5f
Induction of cellular antioxidant defense by amifostine improves ventilator-induced lung injury
Abstract
Objectives: To test the hypothesis that preconditioning animals with amifostine improves ventilator-induced lung injury via induction of antioxidant defense enzymes. Mechanical ventilation at high tidal volume induces reactive oxygen species production and oxidative stress in the lung, which plays a major role in the pathogenesis of ventilator-induced lung injury. Amifostine attenuates oxidative stress and improves lipopolysaccharide-induced lung injury by acting as a direct scavenger of reactive oxygen and nitrogen species. This study tested effects of chronic amifostine administration on parameters of oxidative stress, lung barrier function, and inflammation associated with ventilator-induced lung injury.
Design: Randomized and controlled laboratory investigation in mice and cell culture.
Setting: University laboratory.
Subjects: C57BL/6J mice.
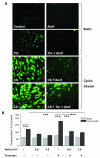
Interventions: Mice received once-daily dosing with amifostine (10-100 mg/kg, intraperitoneal injection) 3 days consecutively before high tidal volume ventilation (30 mL/kg, 4 hrs) at day 4. Pulmonary endothelial cell cultures were exposed to pathologic cyclic stretching (18% equibiaxial stretch) and thrombin in a previously verified two-hit model of in vitro ventilator-induced lung injury.
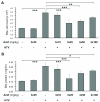
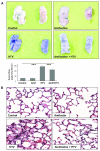
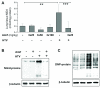
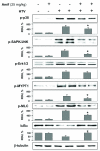
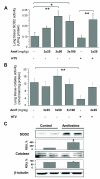
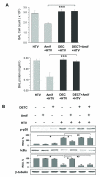
Measurements and main results: Three-day amifostine preconditioning before high tidal volume attenuated high tidal volume-induced protein and cell accumulation in the alveolar space judged by bronchoalveolar lavage fluid analysis, decreased Evans Blue dye extravasation into the lung parenchyma, decreased biochemical parameters of high tidal volume-induced tissue oxidative stress, and inhibited high tidal volume-induced activation of redox-sensitive stress kinases and nuclear factor-kappa B inflammatory cascade. These protective effects of amifostine were associated with increased superoxide dismutase 2 expression and increased superoxide dismutase and catalase enzymatic activities in the animal and endothelial cell culture models of ventilator-induced lung injury.
Conclusions: Amifostine preconditioning activates lung tissue antioxidant cell defense mechanisms and may be a promising strategy for alleviation of ventilator-induced lung injury in critically ill patients subjected to extended mechanical ventilation.
Figures
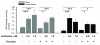

Comment in
-
Central role of oxidative stress and its signaling pathways in causing and preventing acute lung injury.Crit Care Med. 2011 Dec;39(12):2776-7. doi: 10.1097/CCM.0b013e31822b3a00. Crit Care Med. 2011. PMID: 22094514 No abstract available.
-
Low-dose antioxidant is sufficient to regulate pulmonary redox equilibration.Crit Care Med. 2012 May;40(5):1693-4. doi: 10.1097/CCM.0b013e318246b84e. Crit Care Med. 2012. PMID: 22511172 No abstract available.
References
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005 Oct 20;353(16):1685–93. - PubMed
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1334–49. - PubMed
-
- Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. Jama. 1999 Jul 7;282(1):54–61. - PubMed
-
- Villar J, Flores C, Mendez-Alvarez S. Genetic susceptibility to acute lung injury. Crit Care Med. 2003 Apr;31(4 Suppl):S272–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

