Impaired adenosine-5'-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion
- PMID: 21765360
- PMCID: PMC3196852
- DOI: 10.1097/CCM.0b013e318225754f
Impaired adenosine-5'-triphosphate release from red blood cells promotes their adhesion to endothelial cells: a mechanism of hypoxemia after transfusion
Abstract
Objective: Transfusion of red blood cells has been linked to disappointing clinical outcomes in the critically ill, but specific mechanisms of organ dysfunction after transfusion remain poorly understood. We tested the hypothesis that red blood cell storage impairs the ability of red blood cells to release adenosine-5'-triphosphate and that impaired adenosine-5'-triphosphate release was injurious in vivo, in part through increased red blood cell adhesion.
Design: Prospective, controlled, mechanistic study.
Setting: University research laboratory.
Subjects: Human and mouse blood donors; nude mouse transfusion recipients.
Interventions: Manipulation of adenosine-5'-triphosphate release, supplemental adenosine-5'-triphosphate, and antibodies to red blood cell and endothelial adhesion receptors were used in vitro and in vivo to probe the roles of released adenosine-5'-triphosphate and adhesion in responses to (transfused) red blood cells.
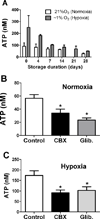
Measurements and main results: The ability of stored red blood cells to release adenosine-5'-triphosphate declined markedly within 14 days after collection despite relatively stable levels of adenosine-5'-triphosphate within the red blood cells. Inhibiting adenosine-5'-triphosphate release promoted the adhesion of stored red blood cells to endothelial cells in vitro and red blood cell sequestration in the lungs of transfused mice in vivo. Unlike transfusion of fresh human red blood cells, stored red blood cell transfusion in mice decreased blood oxygenation and increased extravasation of red blood cells into the lung's alveolar air spaces. Similar findings were seen with transfusion of fresh red blood cells treated with the adenosine-5'-triphosphate release inhibitors glibenclamide and carbenoxolone. These findings were prevented by either coinfusion of an adenosine-5'-triphosphate analog or pretransfusion incubation of the red blood cells with an antibody against the erythrocyte adhesion receptor Landsteiner-Wiener (intercellular adhesion molecule-4).
Conclusions: The normal flow of red blood cells in pulmonary microvessels depends in part on the release of antiadhesive adenosine-5'-triphosphate from red blood cells, and storage-induced deficiency in adenosine-5'-triphosphate release from transfused red blood cells may promote or exacerbate microvascular pathophysiology in the lung, in part through increased red blood cell adhesion.
Conflict of interest statement
Figures






Comment in
-
Losing control over adenosine 5'-triphosphate release: implications for the red blood cell storage lesion.Crit Care Med. 2011 Nov;39(11):2573-4. doi: 10.1097/CCM.0b013e31822a55fa. Crit Care Med. 2011. PMID: 22005234 Free PMC article. No abstract available.
References
-
- Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. - PubMed
-
- Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. - PubMed
-
- Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. - PubMed
-
- Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–1562. - PubMed
-
- Tinmouth A, Fergusson D, Yee IC, et al. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–2027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

