Multiple modalities converge on a common gate to control K2P channel function
- PMID: 21765396
- PMCID: PMC3181481
- DOI: 10.1038/emboj.2011.230
Multiple modalities converge on a common gate to control K2P channel function
Abstract
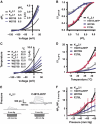
Members of the K(2P) potassium channel family regulate neuronal excitability and are implicated in pain, anaesthetic responses, thermosensation, neuroprotection, and mood. Unlike other potassium channels, K(2P)s are gated by remarkably diverse stimuli that include chemical, thermal, and mechanical modalities. It has remained unclear whether the various gating inputs act through separate or common channel elements. Here, we show that protons, heat, and pressure affect activity of the prototypical, polymodal K(2P), K(2P)2.1 (KCNK2/TREK-1), at a common molecular gate that comprises elements of the pore-forming segments and the N-terminal end of the M4 transmembrane segment. We further demonstrate that the M4 gating element is conserved among K(2P)s and is employed regardless of whether the gating stimuli are inhibitory or activating. Our results define a unique gating mechanism shared by K(2P) family members and suggest that their diverse sensory properties are achieved by coupling different molecular sensors to a conserved core gating apparatus.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


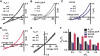


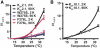

References
-
- Barel O, Shalev SA, Ofir R, Cohen A, Zlotogora J, Shorer Z, Mazor G, Finer G, Khateeb S, Zilberberg N, Birk OS (2008) Maternally inherited Birk Barel mental retardation dysmorphism syndrome caused by a mutation in the genomically imprinted potassium channel KCNK9. Am J Hum Genet 83: 193–199 - PMC - PubMed
-
- Ben-Abu Y, Zhou Y, Zilberberg N, Yifrach O (2009) Inverse coupling in leak and voltage-activated K+ channel gates underlies distinct roles in electrical signaling. Nat Struct Mol Biol 16: 71–79 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

