The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function
- PMID: 21765414
- PMCID: PMC3158818
- DOI: 10.1038/ni.2068
The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function
Abstract
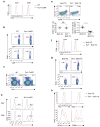
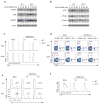
The mechanisms that regulate T cell quiescence are poorly understood. We report that the tumor suppressor Tsc1 established a quiescence program in naive T cells by controlling cell size, cell cycle entry and responses to stimulation of the T cell antigen receptor. Abrogation of quiescence predisposed Tsc1-deficient T cells to apoptosis that resulted in loss of conventional T cells and invariant natural killer T cells. Loss of Tsc1 function dampened in vivo immune responses to bacterial infection. Tsc1-deficient T cells had more activity of the serine-threonine kinase complex mTORC1 but less mTORC2 activity, and activation of mTORC1 was essential for the disruption of immune homeostasis. Therefore, Tsc1-dependent control of mTOR is crucial in actively maintaining the quiescence of naive T cells to facilitate adaptive immune function.
Figures
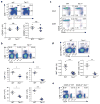

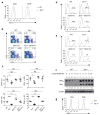
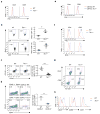
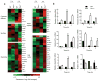

Comment in
-
T cells: mTOR lullabies for naive T cells.Nat Rev Immunol. 2011 Aug 5;11(9):572. doi: 10.1038/nri3047. Nat Rev Immunol. 2011. PMID: 21818125 No abstract available.
-
A 'Tsc, Tsc' keeps the kids quie(scen)t and holds down ROS.Nat Immunol. 2011 Aug 18;12(9):811-2. doi: 10.1038/ni.2092. Nat Immunol. 2011. PMID: 21852776 No abstract available.
References
-
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. - PubMed
-
- Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. - PubMed
-
- Glynne R, Ghandour G, Rayner J, Mack DH, Goodnow CC. B-lymphocyte quiescence, tolerance and activation as viewed by global gene expression profiling on microarrays. Immunol Rev. 2000;176:216–246. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

