The interaction between caveolin-1 and Rho-GTPases promotes metastasis by controlling the expression of alpha5-integrin and the activation of Src, Ras and Erk
- PMID: 21765460
- PMCID: PMC3289793
- DOI: 10.1038/onc.2011.288
The interaction between caveolin-1 and Rho-GTPases promotes metastasis by controlling the expression of alpha5-integrin and the activation of Src, Ras and Erk
Abstract
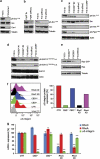
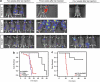
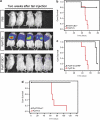
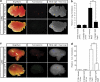
Proteins containing a caveolin-binding domain (CBD), such as the Rho-GTPases, can interact with caveolin-1 (Cav1) through its caveolin scaffold domain. Rho-GTPases are important regulators of p130(Cas), which is crucial for both normal cell migration and Src kinase-mediated metastasis of cancer cells. However, although Rho-GTPases (particularly RhoC) and Cav1 have been linked to cancer progression and metastasis, the underlying molecular mechanisms are largely unknown. To investigate the function of Cav1-Rho-GTPase interaction in metastasis, we disrupted Cav1-Rho-GTPase binding in melanoma and mammary epithelial tumor cells by overexpressing CBD, and examined the loss-of-function of RhoC in metastatic cancer cells. Cancer cells overexpressing CBD or lacking RhoC had reduced p130(Cas) phosphorylation and Rac1 activation, resulting in an inhibition of migration and invasion in vitro. The activity of Src and the activation of its downstream targets FAK, Pyk2, Ras and extracellular signal-regulated kinase (Erk)1/2 were also impaired. A reduction in α5-integrin expression, which is required for binding to fibronectin and thus cell migration and survival, was observed in CBD-expressing cells and cells lacking RhoC. As a result of these defects, CBD-expressing melanoma cells had a reduced ability to metastasize in recipient mice, and impaired extravasation and survival in secondary sites in chicken embryos. Our data indicate that interaction between Cav1 and Rho-GTPases (most likely RhoC but not RhoA) promotes metastasis by stimulating α5-integrin expression and regulating the Src-dependent activation of p130(Cas)/Rac1, FAK/Pyk2 and Ras/Erk1/2 signaling cascades.
Figures


References
-
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. - PubMed
-
- Brabek J, Constancio SS, Siesser PF, Shin NY, Pozzi A, Hanks SK. Crk-associated substrate tyrosine phosphorylation sites are critical for invasion and metastasis of SRC-transformed cells. Mol Cancer Res. 2005;3:307–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

