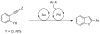Tandem Cycloisomerization/Suzuki Coupling of Arylethynyl MIDA Boronates
- PMID: 21765556
- PMCID: PMC3134963
- DOI: 10.1016/j.tet.2011.04.011
Tandem Cycloisomerization/Suzuki Coupling of Arylethynyl MIDA Boronates
Abstract
A tandem gold-catalyzed cycloisomerization/Suzuki cross coupling sequence involving arylethynyl-N-methyliminodiacetic acid boronates is described. Combining the mildness of homogeneous gold catalysis with the versatility of N-methyliminodiacetic acid (MIDA) boronates, this tandem two-step method enables the rapid assembly of various aryl-substituted heterocycles without having to isolate or purify any heterocyclic MIDA boronate intermediates. Another major advantage of this method is that a wide range of heterocycles bearing different aryl groups may be made from a single MIDA boronate alkyne precursor.
Figures
References
-
- De Luca L, Nieddu G, Porcheddu A, Giacomelli G. Curr Med Chem. 2009;16:1–20. - PubMed
- Bräse S, Gil C, Knepper K. Bioorg Med Chem. 2002;10:2415–2437. - PubMed
- Mor M, Spadoni G, Di Giacomo B, Diamantini G, Bedini A, Tarzia G, Plazzi PV, Rivara S, Nonno R, Lucini V, Pannacci M, Fraschini F, Stankov BM. Bioorg Med Chem. 2001;10:1045–1057. - PubMed
- Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893–930. - PubMed
- Engler TA, LaTessa KO, Iyengar R, Chai W, Agrios K. Bioorg Med Chem. 1996;4:1755–1769. - PubMed
- Dai JR, Hallock YF, Cardellina JH, Boyd M. J Nat Prod. 1998;61:351–353. - PubMed
- Diyasena MNC, Sotheeswaran S, Surendrakumar S, Balasubramanian S, Bokel M, Kraus W. J Chem Soc Perkin Trans. 1985;1:1807–1809.
- Dean FM. In: The Total Synthesis of Natural Products. ApSimon J, editor. Vol. 1 Wiley; New York: 1973.
-
- Katritzky AR, Li J, Stevens CV. J Org Chem. 1995;60:3401–3404.
- Kraus GA, Guo H. Org Lett. 2008;10:3061–3063. - PubMed
- Taber DF, Tian W. J Am Chem Soc. 2006;128:1058–1059. - PMC - PubMed
- Fang YQ, Lautens M. Org Lett. 2005;7:3549–3552. - PubMed
- Gribble GW. J Chem Soc Perkin Trans 1. 2000;7:1045–1075.
- Yue D, Yao T, Larock RC. J Org Chem. 2006;71:62–69. - PMC - PubMed
- Kumar MP, Liu RS. J Org Chem. 2006;71:4951–4955. - PubMed
-
-
For examples of boron and silicon containing substrates in gold-catalyzed cycloisomerization reactions see: Park S, Lee D. J Am Chem Soc. 2006;128:10664–10665.Horino W, Luzung MR, Toste FD. J Am Chem Soc. 2006;128:11364–11365.Matsuda T, Kadowaki S, Yamaguchi Y, Murakami M. Chem Commun. 2008:2744–2746.Lee JCH, Hall DG. Tetrahedron Lett. 2011;52:321–324.
-
-
- Gillis EP, Burke MD. Aldrichimica Acta. 2009;42:17–27. - PMC - PubMed
- Lee SJ, Anderson TM, Burke MD. Angew Chem Int Ed. 2010;49:8860–8863. - PMC - PubMed
- Woerly EM, Cherney AH, Davis EK, Burke MD. J Am Chem Soc. 2010;132:6941–6943. - PMC - PubMed
- Dick GR, Knapp DM, Gillis EP, Burke MD. Org Lett. 2010;12:2314–2317. - PMC - PubMed
- Knapp DM, Gillis EP, Burke MD. J Am Chem Soc. 2009;131:6961–6963. - PMC - PubMed
-
- Struble JR, Lee SJ, Burke MD. Tetrahedron. 2010;66:4710–4718.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources