Thrombospondin-1: multiple paths to inflammation
- PMID: 21765615
- PMCID: PMC3134184
- DOI: 10.1155/2011/296069
Thrombospondin-1: multiple paths to inflammation
Abstract
Inflammation is a defensive process against tissue injury. Once this self-protective strategy is initiated, an effective resolution of the process is crucial to avoid major and unnecessary tissue damage. If the underlying event inducing inflammation is not addressed and homeostasis is not restored, this process can become chronic and lead to angiogenesis and carcinogenesis. Thrombospondin-1 (TSP-1) is a matricellular protein involved in angiogenesis, cancer, and inflammation. The effects of TSP-1 have been studied in many preclinical tumor models, and mimetic peptides are being tested in cancer clinical trials. However, the molecular mechanisms explaining its role in inflammatory processes are not well understood. This paper will discuss the role of TSP-1 in inflammation and its interaction with key receptors that may explain its functions in that process. Recent literature will be reviewed showing novel mechanisms by which this multifaceted protein could modulate the inflammatory process and impact its resolution.
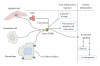
Figures


References
-
- Lawler J, Slayter H, Coligan JE. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. Journal of Biological Chemistry. 1978;253(23):8609–8616. - PubMed
-
- Wight TN, Raugi G, Mumby SM, Bornstein P. Light microscopic immunolocation of thrombospondin in human tissues. Journal of Histochemistry and Cytochemistry. 1985;33(4):295–302. - PubMed
-
- Naganuma H, Satoh H, Asahara T, et al. Quantification of thrombospondin-1 secretion and expression of αvβ3 and α3β1 integrins and syndecan-1 as cell-surface receptors for thrombospondin-1 in malignant glioma cells. Journal of Neuro-Oncology. 2004;70(3):309–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

