Defining the specificity of cotranslationally acting chaperones by systematic analysis of mRNAs associated with ribosome-nascent chain complexes
- PMID: 21765803
- PMCID: PMC3134442
- DOI: 10.1371/journal.pbio.1001100
Defining the specificity of cotranslationally acting chaperones by systematic analysis of mRNAs associated with ribosome-nascent chain complexes
Abstract
Polypeptides exiting the ribosome must fold and assemble in the crowded environment of the cell. Chaperones and other protein homeostasis factors interact with newly translated polypeptides to facilitate their folding and correct localization. Despite the extensive efforts, little is known about the specificity of the chaperones and other factors that bind nascent polypeptides. To address this question we present an approach that systematically identifies cotranslational chaperone substrates through the mRNAs associated with ribosome-nascent chain-chaperone complexes. We here focused on two Saccharomyces cerevisiae chaperones: the Signal Recognition Particle (SRP), which acts cotranslationally to target proteins to the ER, and the Nascent chain Associated Complex (NAC), whose function has been elusive. Our results provide new insights into SRP selectivity and reveal that NAC is a general cotranslational chaperone. We found surprising differential substrate specificity for the three subunits of NAC, which appear to recognize distinct features within nascent chains. Our results also revealed a partial overlap between the sets of nascent polypeptides that interact with NAC and SRP, respectively, and showed that NAC modulates SRP specificity and fidelity in vivo. These findings give us new insight into the dynamic interplay of chaperones acting on nascent chains. The strategy we used should be generally applicable to mapping the specificity, interplay, and dynamics of the cotranslational protein homeostasis network.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
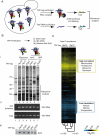
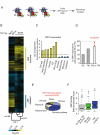
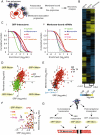


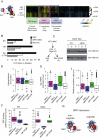
Comment in
-
SRP and NAC: guiding a nascent protein's first steps.PLoS Biol. 2011 Jul;9(7):e1001103. doi: 10.1371/journal.pbio.1001103. Epub 2011 Jul 12. PLoS Biol. 2011. PMID: 21765805 Free PMC article. No abstract available.
References
-
- Albanese V, Yam A. Y, Baughman J, Parnot C, Frydman J. Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell. 2006;124:75–88. - PubMed
-
- Brown J. D, Ng D. T, Ogg S. C, Walter P. Targeting pathways to the endoplasmic reticulum membrane. Cold Spring Harb Symp Quant Biol. 1995;60:23–30. - PubMed
-
- Wegrzyn R. D, Hofmann D, Merz F, Nikolay R, Rauch T, et al. A conserved motif is prerequisite for the interaction of NAC with ribosomal protein L23 and nascent chains. J Biol Chem. 2006;281:2847–2857. - PubMed
-
- Jungnickel B, Rapoport T. a. A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell. 1995;82:261–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

