Can Functional Magnetic Resonance Imaging Improve Success Rates in CNS Drug Discovery?
- PMID: 21765857
- PMCID: PMC3134334
- DOI: 10.1517/17460441.2011.584529
Can Functional Magnetic Resonance Imaging Improve Success Rates in CNS Drug Discovery?
Abstract
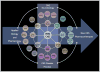
INTRODUCTION: The bar for developing new treatments for CNS disease is getting progressively higher and fewer novel mechanisms are being discovered, validated and developed. The high costs of drug discovery necessitate early decisions to ensure the best molecules and hypotheses are tested in expensive late stage clinical trials. The discovery of brain imaging biomarkers that can bridge preclinical to clinical CNS drug discovery and provide a 'language of translation' affords the opportunity to improve the objectivity of decision-making. AREAS COVERED: This review discusses the benefits, challenges and potential issues of using a science based biomarker strategy to change the paradigm of CNS drug development and increase success rates in the discovery of new medicines. The authors have summarized PubMed and Google Scholar based publication searches to identify recent advances in functional, structural and chemical brain imaging and have discussed how these techniques may be useful in defining CNS disease state and drug effects during drug development. EXPERT OPINION: The use of novel brain imaging biomarkers holds the bold promise of making neuroscience drug discovery smarter by increasing the objectivity of decision making thereby improving the probability of success of identifying useful drugs to treat CNS diseases. Functional imaging holds the promise to: (1) define pharmacodynamic markers as an index of target engagement (2) improve translational medicine paradigms to predict efficacy; (3) evaluate CNS efficacy and safety based on brain activation; (4) determine brain activity drug dose-response relationships and (5) provide an objective evaluation of symptom response and disease modification.
Conflict of interest statement
L Becerra has no conflicts of interest and has received no payment in the preparation of this manuscript. D Borsook is supported by an NIH grant (NINDS K24 NS064050) and declares no other conflict of interest. R Hargreaves is a full time employee of Merck Research Laboratories.
Figures



References
-
- Kaitin KI, Dimasi JA. Pharmaceutical innovation in the 21st century: new drug approvals in the first decade, 2000–2009. Clin Pharmacol Ther. 2011;89(2):183–8. Defines the problems related to the productivity of drug development. - PubMed
-
- Honig P, Lalonde R. The economics of drug development: a grim reality and a role for clinical pharmacology. Clin Pharmacol Ther. 2010;87(3):247–51. - PubMed
-
- Hampel H, Frank R, Broich K, et al. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9(7):560–74. A useful perspective on the benefits of biomarkers in CNS drug development. - PubMed
-
- Vastag M, Keseru GM. Current in vitro and in silico models of blood-brain barrier penetration: a practical view. Curr Opin Drug Discov Devel. 2009;12(1):115–24. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
