IgE-mediated enhancement of CD4+ T cell responses in mice requires antigen presentation by CD11c+ cells and not by B cells
- PMID: 21765910
- PMCID: PMC3130775
- DOI: 10.1371/journal.pone.0021760
IgE-mediated enhancement of CD4+ T cell responses in mice requires antigen presentation by CD11c+ cells and not by B cells
Abstract
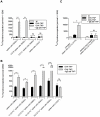
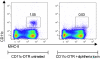
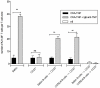
IgE antibodies, administered to mice together with their specific antigen, enhance antibody and CD4(+) T cell responses to this antigen. The effect is dependent on the low affinity receptor for IgE, CD23, and the receptor must be expressed on B cells. In vitro, IgE-antigen complexes are endocytosed via CD23 on B cells, which subsequently present the antigen to CD4(+) T cells. This mechanism has been suggested to explain also IgE-mediated enhancement of immune responses in vivo. We recently found that CD23(+) B cells capture IgE-antigen complexes in peripheral blood and rapidly transport them to B cell follicles in the spleen. This provides an alternative explanation for the requirement for CD23(+) B cells. The aim of the present study was to determine whether B-cell mediated antigen presentation of IgE-antigen complexes explains the enhancing effect of IgE on immune responses in vivo. The ability of spleen cells, taken from mice 1-4 h after immunization with IgE-antigen, to present antigen to specific CD4(+) T cells was analyzed. Antigen presentation was intact when spleens were depleted of CD19(+) cells (i.e., primarily B cells) but was severely impaired after depletion of CD11c(+) cells (i.e., primarily dendritic cells). In agreement with this, the ability of IgE to enhance proliferation of CD4(+) T cells was abolished in CD11c-DTR mice conditionally depleted of CD11c(+) cells. Finally, the lack of IgE-mediated enhancemen of CD4(+) T cell responses in CD23(-/-) mice could be rescued by transfer of MHC-II-compatible as well as by MHC-II-incompatible CD23(+) B cells. These findings argue against the idea that IgE-mediated enhancement of specific CD4(+) T cell responses in vivo is caused by increased antigen presentation by B cells. A model where CD23(+) B cells act as antigen transporting cells, delivering antigen to CD11c(+) cells for presentation to T cells is consistent with available experimental data.
Conflict of interest statement
Figures

Similar articles
-
IgE-mediated enhancement of CD4(+) T cell responses requires antigen presentation by CD8α(-) conventional dendritic cells.Sci Rep. 2016 Jun 16;6:28290. doi: 10.1038/srep28290. Sci Rep. 2016. PMID: 27306570 Free PMC article.
-
A novel B cell-mediated transport of IgE-immune complexes to the follicle of the spleen.J Immunol. 2008 May 15;180(10):6604-10. doi: 10.4049/jimmunol.180.10.6604. J Immunol. 2008. PMID: 18453579
-
A novel recycling mechanism of native IgE-antigen complexes in human B cells facilitates transfer of antigen to dendritic cells for antigen presentation.J Allergy Clin Immunol. 2018 Aug;142(2):557-568.e6. doi: 10.1016/j.jaci.2017.09.024. Epub 2017 Oct 23. J Allergy Clin Immunol. 2018. PMID: 29074459
-
The role of CD23 in the regulation of allergic responses.Allergy. 2021 Jul;76(7):1981-1989. doi: 10.1111/all.14724. Epub 2021 Jan 16. Allergy. 2021. PMID: 33378583 Free PMC article. Review.
-
IgG- and IgE-mediated antigen presentation on MHC class II.Immunol Lett. 2004 Mar 29;92(1-2):33-8. doi: 10.1016/j.imlet.2003.09.015. Immunol Lett. 2004. PMID: 15081524 Review.
Cited by
-
CD4+ T-cell-dependent differentiation of CD23+ follicular B cells contributes to the pulmonary pathology in a primary Sjögren's syndrome mouse model.Front Immunol. 2023 Jul 5;14:1217492. doi: 10.3389/fimmu.2023.1217492. eCollection 2023. Front Immunol. 2023. PMID: 37475871 Free PMC article.
-
IgG3-antigen complexes are deposited on follicular dendritic cells in the presence of C1q and C3.Sci Rep. 2017 Jul 14;7(1):5400. doi: 10.1038/s41598-017-05704-3. Sci Rep. 2017. PMID: 28710441 Free PMC article.
-
Maternal IgE Influence on Fetal and Infant Health.Immunol Rev. 2025 May;331(1):e70029. doi: 10.1111/imr.70029. Immunol Rev. 2025. PMID: 40281548 Free PMC article. Review.
-
Signaling by Antibodies: Recent Progress.Annu Rev Immunol. 2017 Apr 26;35:285-311. doi: 10.1146/annurev-immunol-051116-052433. Annu Rev Immunol. 2017. PMID: 28446061 Free PMC article. Review.
-
Antibody feedback regulation.Immunol Rev. 2024 Nov;328(1):126-142. doi: 10.1111/imr.13377. Epub 2024 Aug 23. Immunol Rev. 2024. PMID: 39180190 Free PMC article. Review.
References
-
- Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol. 2000;18:709–737. - PubMed
-
- von Behring E, Wernicke E. Über Immunisierung und Heilung von Versuchstieren bei der Diphterie. Z Hyg Infektionskrankheit. 1892;12:10–44.
-
- Bowman JM. The prevention of Rh immunization. Transfus Med Rev. 1988;2:129–150. - PubMed
-
- Wernersson S, Karlsson M, Dahlström J, Mattsson R, Verbeek JS, et al. IgG-mediated enhancement of Ab responses is low in FcRγ chain deficient mice and increased in FcγRII deficient mice. J Immunol. 1999;163:618–622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

