Does HAART efficacy translate to effectiveness? Evidence for a trial effect
- PMID: 21765918
- PMCID: PMC3135599
- DOI: 10.1371/journal.pone.0021824
Does HAART efficacy translate to effectiveness? Evidence for a trial effect
Abstract
Background: Patients who participate in clinical trials may experience better clinical outcomes than patients who initiate similar therapy within clinical care (trial effect), but no published studies have evaluated a trial effect in HIV clinical trials.
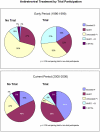
Methods: To examine a trial effect we compared virologic suppression (VS) among patients who initiated HAART in a clinical trial versus in routine clinical care. VS was defined as a plasma HIV RNA ≤ 400 copies/ml at six months after HAART initiation and was assessed within strata of early (1996-99) or current (2000-06) HAART periods. Risk ratios (RR) were estimated using binomial models.
Results: Of 738 persons initiating HAART, 30.6% were women, 61.7% were black, 30% initiated therapy in a clinical trial and 67% (n = 496) had an evaluable six month HIV RNA result. HAART regimens differed between the early and current periods (p < 0.001); unboosted PI regimens (55.6%) were more common in the early and NNRTI regimens (46.4%) were more common in the current period. Overall, 78% (95%CI 74, 82%) of patients achieved VS and trial participants were 16% more likely to achieve VS (unadjusted RR 1.16, 95%CI 1.06, 1.27). Comparing trial to non-trial participants, VS differed by study period. In the early period, trial participants initiating HAART were significantly more likely to achieve VS than non-trial participants (adjusted RR 1.33; 95%CI 1.15, 1.54), but not in the current period (adjusted RR 0.98; 95%CI 0.87, 1.11).
Conclusions: A clear clinical trial effect on suppression of HIV replication was observed in the early HAART period but not in the current period.
Conflict of interest statement
Figures
References
-
- Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J Clin Epidemiol. 2001;54:217–224. - PubMed
-
- Lantos JD. The “inclusion benefit” in clinical trials. J Pediatr. 1999;134:130–131. - PubMed
-
- Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363:263–270. - PubMed
-
- Silverman WA. Disclosing the “inclusion benefit”. J Perinatol. 2002;22:261–262. - PubMed
-
- ECRI Evidence Report: Patients' reasons for participating in clinical trials and effect of trial participation on patient outcomes. 2002. Available at: https://www.ecri.org/Documents/Clinical_Trials_Patient_Guide_Evidence_Re.... Accessed June 27, 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous