Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways
- PMID: 21765922
- PMCID: PMC3134470
- DOI: 10.1371/journal.pone.0021891
Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways
Abstract
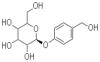
Background: Microglial activation plays an important role in neurodegenerative diseases by producing several proinflammatory enzymes and proinflammatory cytokines. The phenolic glucoside gastrodin, a main constituent of a Chinese herbal medicine, has been known to display anti-inflammatory properties. The current study investigates the potential mechanisms whereby gastrodin affects the expression of potentially pro-inflammatory proteins by cultured murine microglial BV-2 cells stimulated with lipopolysaccharide (LPS).
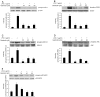
Methodology/principal findings: BV-2 cells were pretreated with gastrodin (30, 40, and 60 µM) for 1 h and then stimulated with LPS (1 µg/ml) for another 4 h. The effects on proinflammatory enzymes, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), and proinflammatory cytokines, tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β), are analysed by double-immunofluorescence labeling and RT-PCR assay. To reveal the mechanisms of action of gastrodin we investigated the involvement of mitogen-activated protein kinases (MAPKs) cascades and their downstream transcription factors, nuclear factor-κB (NF-κB) and cyclic AMP-responsive element (CRE)-binding protein (CREB). Gastrodin significantly reduced the LPS-induced protein and mRNA expression levels of iNOS, COX-2, TNF-α, IL-1β and NF-κB. LPS (1 µg/ml, 30 min)-induced phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal protein kinase (JNK) and p38 mitogen-activated protein kinase (p38 MAPK) and this was inhibited by pretreatment of BV-2 cells with different concentrations of gastrodin (30, 40, and 60 µM). In addition, gastrodin blocked LPS-induced phosphorylation of inhibitor κB-α (IκB-α) (and hence the activation of NF-κB) and of CREB, respectively.
Conclusion and implications: This study indicates that gastrodin significantly attenuate levels of neurotoxic proinflammatory mediators and proinflammatory cytokines by inhibition of the NF-κB signaling pathway and phosphorylation of MAPKs in LPS-stimulated microglial cells. Arising from the above, we suggest that gastrodin has a potential as an anti-inflammatory drug candidate in neurodegenerative diseases.
Conflict of interest statement
Figures
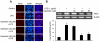

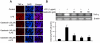


References
-
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. - PubMed
-
- Nakajima K, Kohsaka S. Functional roles of microglia in the central nervous system. Hum Cell. 1998;11:141–155. - PubMed
-
- Suzumura A, Takeuchi H, Zhang G, Kuno R, Mizuno T. Roles of glia-derived cytokines on neuronal degeneration and regeneration. Ann N Y Acad Sci. 2006;1088:219–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

