Vasoactive intestinal peptide signaling axis in human leukemia
- PMID: 21765981
- PMCID: PMC3135862
- DOI: 10.4331/wjbc.v2.i6.146
Vasoactive intestinal peptide signaling axis in human leukemia
Abstract
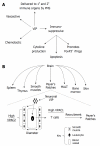
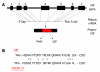
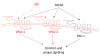
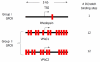
The vasoactive intestinal peptide (VIP) signaling axis constitutes a master "communication coordinator" between cells of the nervous and immune systems. To date, VIP and its two main receptors expressed in T lymphocytes, vasoactive intestinal peptide receptor (VPAC)1 and VPAC2, mediate critical cellular functions regulating adaptive immunity, including arresting CD4 T cells in G(1) of the cell cycle, protection from apoptosis and a potent chemotactic recruiter of T cells to the mucosa associated lymphoid compartment of the gastrointestinal tissues. Since the discovery of VIP in 1970, followed by the cloning of VPAC1 and VPAC2 in the early 1990s, this signaling axis has been associated with common human cancers, including leukemia. This review highlights the present day knowledge of the VIP ligand and its receptor expression profile in T cell leukemia and cell lines. Also, there will be a discussion describing how the anti-leukemic DNA binding transcription factor, Ikaros, regulates VIP receptor expression in primary human CD4 T lymphocytes and T cell lymphoblastic cell lines (e.g. Hut-78). Lastly, future goals will be mentioned that are expected to uncover the role of how the VIP signaling axis contributes to human leukemogenesis, and to establish whether the VIP receptor signature expressed by leukemic blasts can provide therapeutic and/or diagnostic information.
Keywords: Cancer; Epigenetics; Hut-78; Ikaros; Neuropeptides.
Figures


Similar articles
-
Selective gene expression and activation-dependent regulation of vasoactive intestinal peptide receptor type 1 and type 2 in human T cells.J Immunol. 2001 Feb 15;166(4):2522-30. doi: 10.4049/jimmunol.166.4.2522. J Immunol. 2001. PMID: 11160313
-
A cloned frog vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating polypeptide receptor exhibits pharmacological and tissue distribution characteristics of both VPAC1 and VPAC2 receptors in mammals.Endocrinology. 1999 Mar;140(3):1285-93. doi: 10.1210/endo.140.3.6576. Endocrinology. 1999. PMID: 10067855
-
Alterations in the relative abundance of the vasoactive intestinal peptide receptors (VPAC1 and VPAC2) and functions in uterine contractility during inflammation.Anim Reprod Sci. 2021 Feb;225:106680. doi: 10.1016/j.anireprosci.2020.106680. Epub 2020 Dec 16. Anim Reprod Sci. 2021. PMID: 33388613
-
Regulatory effects of vasoactive intestinal peptide on cytokine production in central and peripheral lymphoid organs.Adv Neuroimmunol. 1996;6(1):61-74. doi: 10.1016/s0960-5428(96)00007-1. Adv Neuroimmunol. 1996. PMID: 8790782 Review.
-
Vasoactive intestinal peptide-mediated Th17 differentiation: an expanding spectrum of vasoactive intestinal peptide effects in immunity and autoimmunity.Ann N Y Acad Sci. 2008 Nov;1144:83-9. doi: 10.1196/annals.1418.020. Ann N Y Acad Sci. 2008. PMID: 19076367 Review.
Cited by
-
Administration of a vasoactive intestinal peptide antagonist enhances the autologous anti-leukemia T cell response in murine models of acute leukemia.Oncoimmunology. 2017 Mar 16;6(5):e1304336. doi: 10.1080/2162402X.2017.1304336. eCollection 2017. Oncoimmunology. 2017. PMID: 28638725 Free PMC article.
-
Messenger RNA Gene Expression Screening of VIP and PACAP Neuropeptides and Their Endogenous Receptors in Ruminants.Biology (Basel). 2022 Oct 15;11(10):1512. doi: 10.3390/biology11101512. Biology (Basel). 2022. PMID: 36290416 Free PMC article.
-
The G Protein-Coupled Receptor, VPAC1, Mediates Vasoactive Intestinal Peptide-Dependent Functional Homeostasis of the Gut Microbiota.Gastro Hep Adv. 2022;1(2):253-264. doi: 10.1016/j.gastha.2021.11.005. Epub 2022 Mar 15. Gastro Hep Adv. 2022. PMID: 36910129 Free PMC article.
-
Ikaros in hematopoiesis and leukemia.World J Biol Chem. 2011 Jun 26;2(6):105-7. doi: 10.4331/wjbc.v2.i6.105. World J Biol Chem. 2011. PMID: 21765974 Free PMC article.
-
Vasoactive Intestinal Peptide Deficiency Is Associated With Altered Gut Microbiota Communities in Male and Female C57BL/6 Mice.Front Microbiol. 2019 Dec 2;10:2689. doi: 10.3389/fmicb.2019.02689. eCollection 2019. Front Microbiol. 2019. PMID: 31849864 Free PMC article.
References
-
- Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217–1218. - PubMed
-
- Cutz E, Chan W, Track NS, Goth A, Said SI. Release of vasoactive intestinal polypeptide in mast cells by histamine liberators. Nature. 1978;275:661–662. - PubMed
-
- O'Dorisio MS, O'Dorisio TM, Cataland S, Balcerzak SP. Vasoactive intestinal polypeptide as a biochemical marker for polymorphonuclear leukocytes. J Lab Clin Med. 1980;96:666–672. - PubMed
-
- Aliakbari J, Sreedharan SP, Turck CW, Goetzl EJ. Selective localization of vasoactive intestinal peptide and substance P in human eosinophils. Biochem Biophys Res Commun. 1987;148:1440–1445. - PubMed
-
- Delgado M, Martínez C, Leceta J, Garrido E, Gomariz RP. Differential VIP and VIP1 receptor gene expression in rat thymocyte subsets. Peptides. 1996;17:803–807. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

