Surveying protein structure and function using bis-arsenical small molecules
- PMID: 21766813
- PMCID: PMC3312748
- DOI: 10.1021/ar2001028
Surveying protein structure and function using bis-arsenical small molecules
Abstract
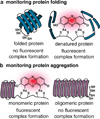
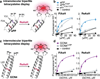
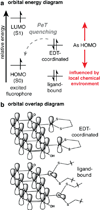
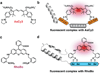
Exploration across the fields of biology, chemical biology, and medicine has led to an increasingly complex, albeit incomplete, view of the interactions that drive life's processes. The ability to monitor and track the movement, activity, and interactions of biomolecules in living cells is an essential part of this investigation. In our laboratory, we have endeavored to develop tools that are capable not only of monitoring protein localization but also reporting on protein structure and function. Central to our efforts is a new strategy, bipartite tetracysteine display, that relies on the specific and high-affinity interaction between a fluorogenic, bis-arsenical small molecule and a unique protein sequence, conformation, or assembly. In 1998, a small-molecule analogue of fluorescein with two arsenic atoms, FlAsH, was shown by Tsien and coworkers to fluoresce upon binding to a linear amino acid sequence, Cys-Cys-Arg-Glu-Cys-Cys. Later work demonstrated that substituting Pro-Gly for Arg-Glu optimized both binding and fluorescence yield. Our strategy of bipartite tetracysteine display emanated from the idea that it would be possible to replace the intervening Pro-Gly dipeptide in this sequence with a protein or protein partnership, provided the assembled protein fold successfully reproduced the approximate placement of the two Cys-Cys pairs. In this Account, we describe our recent progress in this area, with an emphasis on the fundamental concepts that underlie the successful use of bis-arsenicals such as FlAsH and the related ReAsH for bipartite display experiments. In particular, we highlight studies that have explored how broadly bipartite tetracysteine display can be employed and that have navigated the conformational boundary conditions favoring success. To emphasize the utility of these principles, we outline two recently reported applications of bipartite tetracysteine display. The first is a novel, encodable, selective, Src kinase sensor that lacks fluorescent proteins but possesses a fluorescent readout exceeding that of most sensors based on Förster resonance energy transfer (FRET). The second is a unique method, called complex-edited electron microscopy (CE-EM), that facilitates visualization of protein-protein complexes with electron microscopy. Exciting as these applications may be, the continued development of small-molecule tools with improved utility in living cells, let alone in vivo, will demand a more nuanced understanding of the fundamental photophysics that lead to fluorogenicity, as well as creative approaches toward the synthesis and identification of new and orthogonal dye-tag pairs that can be applied facilely in tandem. We describe one example of a dye-sequence tag pair that is chemically distinct from bis-arsenical chemistry. Through further effort, we expect that that bipartite tetracysteine display will find successful use in the study of sophisticated biological questions that are essential to the fields of biochemistry and biology as well as to our progressive understanding of human disease.
© 2011 American Chemical Society
Figures



References
-
- Jares-Erijman EA, Jovin TM. Imaging molecular interactions in living cells by FRET microscopy. Curr. Opin. Chem. Biol. 2006;10(5):409–416. - PubMed
-
- Medintz IL, Mattoussi H. Quantum dot-based resonance energy transfer and its growing application in biology. Phys. Chem. Chem. Phys. 2009;11(1):17–45. - PubMed
-
- Heal WP, Dang TH, Tate EW. Activity-based probes: discovering new biology and new drug targets. Chem. Soc. Rev. 2011;40(1):246–257. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

