Microarray analysis of human adipose-derived stem cells in three-dimensional collagen culture: osteogenesis inhibits bone morphogenic protein and Wnt signaling pathways, and cyclic tensile strain causes upregulation of proinflammatory cytokine regulators and angiogenic factors
- PMID: 21767168
- PMCID: PMC3204199
- DOI: 10.1089/ten.tea.2011.0107
Microarray analysis of human adipose-derived stem cells in three-dimensional collagen culture: osteogenesis inhibits bone morphogenic protein and Wnt signaling pathways, and cyclic tensile strain causes upregulation of proinflammatory cytokine regulators and angiogenic factors
Abstract
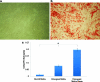
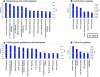
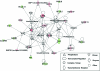
Human adipose-derived stem cells (hASC) have shown great potential for bone tissue engineering. However, the molecular mechanisms underlying this potential are not yet known, in particular the separate and combined effects of three-dimensional (3D) culture and mechanical loading on hASC osteogenesis. Mechanical stimuli play a pivotal role in bone formation, remodeling, and fracture repair. To further understand hASC osteogenic differentiation and response to mechanical stimuli, gene expression profiles of proliferating or osteogenically induced hASC in 3D collagen I culture in the presence and absence of 10% uniaxial cyclic tensile strain were examined using microarray analysis. About 847 genes and 95 canonical pathways were affected during osteogenesis of hASC in 3D culture. Pathway analysis indicated the potential roles of Wnt/β-catenin signaling, bone morphogenic protein (BMP) signaling, platelet-derived growth factor (PDGF) signaling, and insulin-like growth factor 1 (IGF-1) signaling in hASC during osteogenic differentiation. Application of 10% uniaxial cyclic tensile strain suggested synergistic effects of strain with osteogenic differentiation media on hASC osteogenesis as indicated by significantly increased calcium accretion of hASC. There was no significant further alteration in the four major pathways (Wnt/β-catenin, BMP, PDGF, and IGF-1). However, 184 transcripts were affected by 10% cyclic tensile strain. Function and network analysis of these transcripts suggested that 10% cyclic tensile strain may play a role during hASC osteogenic differentiation by upregulating two crucial factors in bone regeneration: (1) proinflammatory cytokine regulators interleukin 1 receptor antagonist and suppressor of cytokine signaling 3; (2) known angiogenic inductors fibroblast growth factor 2, matrix metalloproteinase 2, and vascular endothelial growth factor A. This is the first study to investigate the effects of both 3D culture and mechanical load on hASC osteogenic differentiation. A complete microarray analysis investigating both the separate effect of soluble osteogenic inductive factors and the combined effects of chemical and mechanical stimulation was performed on hASC undergoing osteogenic differentiation. We have identified specific genes and pathways associated with mechanical response and osteogenic potential of hASC, thus providing significant information toward improved understanding of our use of hASC for functional bone tissue engineering applications.
Figures


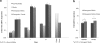
References
-
- Toma J.G. Akhavan M. Fernandes K.J.L. Barnabé-Heider F. Sadikot A. Kaplan D.R. Miller F.D. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778. - PubMed
-
- Kern S. Eichler H. Stoeve J. Kluter H. Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294. - PubMed
-
- Hattori H. Sato M. Masuoka K. Ishihara M. Kikuchi T. Matsui T. Takase B. Ishizuka T. Kikuchi M. Fujikawa K. Osteogenic potential of human adipose tissue-derived stromal cells as an alternative stem cell source. Cells Tissues Organs. 2004;178:2. - PubMed
-
- Halvorsen Y.D.C. Franklin D. Bond A.L. Hitt D.C. Auchter C. Boskey A.L. Paschalis E.P. Wilkison W.O. Gimble J.M. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

