Single-voxel recording of voltage transients in dendritic spines
- PMID: 21767473
- PMCID: PMC3136788
- DOI: 10.1016/j.bpj.2011.06.021
Single-voxel recording of voltage transients in dendritic spines
Abstract
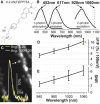
We report sensitive recording of membrane potential in single dendritic spines in cortical neurons within a brain slice using two-photon excitation and a new, fluorinated, intracellularly loaded organic dye, di-2-AN(F)EPPTEA. With a two-photon excitation wavelength of 1060 nm, we achieve voltage sensitivity of >16% change in fluorescence per 100 mV. By targeting single spines in single-voxel recordings, we attain excellent single/noise quality, with back-propagating action potentials (bAPs) visible in single sweeps while recording at 10 kHz. This recording rate allows us to reliably assess fast bAP dynamics on single sweeps including bAP rise times of 0.5 ms. The amplitude and propagation delays of the bAPs are similar among different spines located within the same dendritic region, and this is true despite large differences in spine size. The interregion differences in bAP waveforms in spines vary in relation to their distance from the soma and the caliber of their parent dendrites.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Canepari M., Zecevic D., editors. Membrane Potential Imaging in the Nervous System: Methods and Applications. Springer; New York: 2010.
-
- Fisher J.A.N., Barchi J.R., Salzberg B.M. Two-photon excitation of potentiometric probes enables optical recording of action potentials from mammalian nerve terminals in situ. J. Neurophysiol. 2008;99:1545–1553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

