Defining the role of the tension sensor in the mechanosensitive channel of small conductance
- PMID: 21767486
- PMCID: PMC3136789
- DOI: 10.1016/j.bpj.2011.05.058
Defining the role of the tension sensor in the mechanosensitive channel of small conductance
Abstract
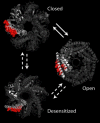
Mutations that alter the phenotypic behavior of the Escherichia coli mechanosensitive channel of small conductance (MscS) have been identified; however, most of these residues play critical roles in the transition between the closed and open states of the channel and are not directly involved in lipid interactions that transduce the tension response. In this study, we use molecular dynamic simulations to predict critical lipid interacting residues in the closed state of MscS. The physiological role of these residues was then investigated by performing osmotic downshock assays on MscS mutants where the lipid interacting residues were mutated to alanine. These experiments identified seven residues in the first and second transmembrane helices as lipid-sensing residues. The majority of these residues are hydrophobic amino acids located near the extracellular interface of the membrane. All of these residues interact strongly with the lipid bilayer in the closed state of MscS, but do not face the bilayer directly in structures associated with the open and desensitized states of the channel. Thus, the position of these residues relative to the lipid membrane appears related to the ability of the channel to sense tension in its different physiological states.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Sukharev S.I., Blount P., Kung C. A large-conductance mechanosensitive channel in E. coli encoded by MscL alone. Nature. 1994;368:265–268. - PubMed
-
- Hurst A.C., Petrov E., Martinac B. MscS, the bacterial mechanosensitive channel of small conductance. Int. J. Biochem. Cell Biol. 2008;40:581–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

