Multiple targets of nitric oxide in the tricarboxylic acid cycle of Salmonella enterica serovar typhimurium
- PMID: 21767810
- PMCID: PMC3142370
- DOI: 10.1016/j.chom.2011.06.004
Multiple targets of nitric oxide in the tricarboxylic acid cycle of Salmonella enterica serovar typhimurium
Abstract
Host nitric oxide (NO⋅) production is important for controlling intracellular bacterial pathogens, including Salmonella enterica serovar Typhimurium, but the underlying mechanisms are incompletely understood. S. Typhmurium 14028s is prototrophic for all amino acids but cannot synthesize methionine (M) or lysine (K) during nitrosative stress. Here, we show that NO⋅-induced MK auxotrophy results from reduced succinyl-CoA availability as a consequence of NO⋅ targeting of lipoamide-dependent lipoamide dehydrogenase (LpdA) activity. LpdA is an essential component of the pyruvate and α-ketoglutarate dehydrogenase complexes. Additional effects of NO⋅ on gene regulation prevent compensatory pathways of succinyl-CoA production. Microarray analysis indicates that over 50% of the transcriptional response of S. Typhimurium to nitrosative stress is attributable to LpdA inhibition. Bacterial methionine transport is essential for virulence in NO⋅-producing mice, demonstrating that NO⋅-induced MK auxotrophy occurs in vivo. These observations underscore the importance of metabolic targets for antimicrobial actions of NO⋅.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
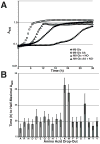
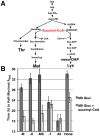

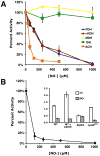
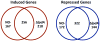

Comment in
-
Another target for NO.Cell Host Microbe. 2011 Jul 21;10(1):1-2. doi: 10.1016/j.chom.2011.07.001. Cell Host Microbe. 2011. PMID: 21767805
Similar articles
-
A Salmonella enterica serovar typhimurium succinate dehydrogenase/fumarate reductase double mutant is avirulent and immunogenic in BALB/c mice.Infect Immun. 2008 Mar;76(3):1128-34. doi: 10.1128/IAI.01226-07. Epub 2007 Dec 17. Infect Immun. 2008. PMID: 18086808 Free PMC article.
-
Methionine biosynthesis and transport are functionally redundant for the growth and virulence of Salmonella Typhimurium.J Biol Chem. 2018 Jun 15;293(24):9506-9519. doi: 10.1074/jbc.RA118.002592. Epub 2018 May 2. J Biol Chem. 2018. PMID: 29720401 Free PMC article.
-
Nitric Oxide Disrupts Zinc Homeostasis in Salmonella enterica Serovar Typhimurium.mBio. 2018 Aug 14;9(4):e01040-18. doi: 10.1128/mBio.01040-18. mBio. 2018. PMID: 30108168 Free PMC article.
-
The metabolic pathways utilized by Salmonella Typhimurium during infection of host cells.Environ Microbiol Rep. 2018 Apr;10(2):140-154. doi: 10.1111/1758-2229.12628. Epub 2018 Feb 23. Environ Microbiol Rep. 2018. PMID: 29411544 Review.
-
Genome Variation and Molecular Epidemiology of Salmonella enterica Serovar Typhimurium Pathovariants.Infect Immun. 2018 Jul 23;86(8):e00079-18. doi: 10.1128/IAI.00079-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29784861 Free PMC article. Review.
Cited by
-
Molecular Underpinnings of Nitrite Effect on CymA-Dependent Respiration in Shewanella oneidensis.Front Microbiol. 2016 Jul 21;7:1154. doi: 10.3389/fmicb.2016.01154. eCollection 2016. Front Microbiol. 2016. PMID: 27493647 Free PMC article.
-
Nitrite modulates aminoglycoside tolerance by inhibiting cytochrome heme-copper oxidase in bacteria.Commun Biol. 2020 May 27;3(1):269. doi: 10.1038/s42003-020-0991-4. Commun Biol. 2020. PMID: 32461576 Free PMC article.
-
Targeted DNA Demethylation: Vectors, Effectors and Perspectives.Biomedicines. 2023 Apr 30;11(5):1334. doi: 10.3390/biomedicines11051334. Biomedicines. 2023. PMID: 37239005 Free PMC article. Review.
-
Manganese import protects Salmonella enterica serovar Typhimurium against nitrosative stress.Metallomics. 2020 Nov 1;12(11):1791-1801. doi: 10.1039/d0mt00178c. Epub 2020 Oct 20. Metallomics. 2020. PMID: 33078811 Free PMC article.
-
The NsrR regulon in nitrosative stress resistance of Salmonella enterica serovar Typhimurium.Mol Microbiol. 2012 Sep;85(6):1179-93. doi: 10.1111/j.1365-2958.2012.08167.x. Epub 2012 Jul 25. Mol Microbiol. 2012. PMID: 22831173 Free PMC article.
References
-
- Bang IS, Liu L, Vazquez-Torres A, Crouch ML, Stamler JS, Fang FC. Maintenance of nitric oxide and redox homeostasis by the Salmonella flavohemoglobin hmp. J Biol Chem. 2006;281:28039–28047. - PubMed
-
- Berggren RE, Wunderlich A, Ziegler E, Schleicher M, Duke RC, Looney D, Fang FC. HIV gp120-specific cell-mediated immune responses in mice after oral immunization with recombinant Salmonella. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:489–495. - PubMed
-
- Boyd EF, Wang FS, Beltran P, Plock SA, Nelson K, Selander RK. Salmonella reference collection B (SARB): strains of 37 serovars of subspecies I. J Gen Microbiol. 1993;139(Pt 6):1125–1132. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

