A novel functional role of collagen glycosylation: interaction with the endocytic collagen receptor uparap/ENDO180
- PMID: 21768090
- PMCID: PMC3173195
- DOI: 10.1074/jbc.M111.266692
A novel functional role of collagen glycosylation: interaction with the endocytic collagen receptor uparap/ENDO180
Abstract
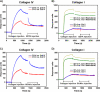
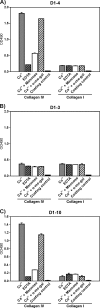
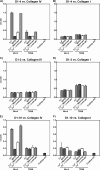
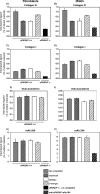
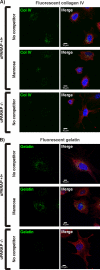
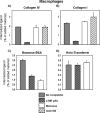
Collagens make up the most abundant component of interstitial extracellular matrices and basement membranes. Collagen remodeling is a crucial process in many normal physiological events and in several pathological conditions. Some collagen subtypes contain specific carbohydrate side chains, the function of which is poorly known. The endocytic collagen receptor urokinase plasminogen activator receptor-associated protein (uPARAP)/Endo180 plays an important role in matrix remodeling through its ability to internalize collagen for lysosomal degradation. uPARAP/Endo180 is a member of the mannose receptor protein family. These proteins all include a fibronectin type II domain and a series of C-type lectin-like domains, of which only a minor part possess carbohydrate recognition activity. At least two of the family members, uPARAP/Endo180 and the mannose receptor, interact with collagens. The molecular basis for this interaction is known to involve the fibronectin type II domain but nothing is known about the function of the lectin domains in this respect. In this study, we have investigated a possible role of the single active lectin domain of uPARAP/Endo180 in the interaction with collagens. By expressing truncated recombinant uPARAP/Endo180 proteins and analyzing their interaction with collagens with high and low levels of glycosylation we demonstrated that this lectin domain interacts directly with glycosylated collagens. This interaction is functionally important because it was found to modulate the endocytic efficiency of the receptor toward highly glycosylated collagens such as basement membrane collagen IV. Surprisingly, this property was not shared by the mannose receptor, which internalized glycosylated collagens independently of its lectin function. This role of modulating its uptake efficiency by a specific receptor is a previously unrecognized function of collagen glycosylation.
Figures

Similar articles
-
The collagen receptor uPARAP/Endo180.Front Biosci (Landmark Ed). 2009 Jan 1;14(6):2103-14. doi: 10.2741/3365. Front Biosci (Landmark Ed). 2009. PMID: 19273187 Review.
-
Complex determinants in specific members of the mannose receptor family govern collagen endocytosis.J Biol Chem. 2014 Mar 14;289(11):7935-47. doi: 10.1074/jbc.M113.512780. Epub 2014 Feb 5. J Biol Chem. 2014. PMID: 24500714 Free PMC article.
-
The non-phagocytic route of collagen uptake: a distinct degradation pathway.J Biol Chem. 2011 Jul 29;286(30):26996-7010. doi: 10.1074/jbc.M110.208033. Epub 2011 Jun 7. J Biol Chem. 2011. PMID: 21652704 Free PMC article.
-
The collagen receptor uPARAP/Endo180 regulates collectins through unique structural elements in its FNII domain.J Biol Chem. 2020 Jul 3;295(27):9157-9170. doi: 10.1074/jbc.RA120.013710. Epub 2020 May 18. J Biol Chem. 2020. PMID: 32424040 Free PMC article.
-
uPARAP/Endo180: a multifaceted protein of mesenchymal cells.Cell Mol Life Sci. 2022 Apr 22;79(5):255. doi: 10.1007/s00018-022-04249-7. Cell Mol Life Sci. 2022. PMID: 35460056 Free PMC article. Review.
Cited by
-
A collagen glucosyltransferase drives lung adenocarcinoma progression in mice.Commun Biol. 2021 Apr 19;4(1):482. doi: 10.1038/s42003-021-01982-w. Commun Biol. 2021. PMID: 33875777 Free PMC article.
-
Comprehensive Mapping and Dynamics of Site-Specific Prolyl-Hydroxylation, Lysyl-Hydroxylation and Lysyl O-Glycosylation of Collagens Deposited in ECM During Zebrafish Heart Regeneration.Front Mol Biosci. 2022 Jun 16;9:892763. doi: 10.3389/fmolb.2022.892763. eCollection 2022. Front Mol Biosci. 2022. PMID: 35782869 Free PMC article.
-
Impact of diabetes mellitus on osteoarthritis: a scoping review on biomarkers.Expert Rev Mol Med. 2024 Apr 12;26:e8. doi: 10.1017/erm.2024.7. Expert Rev Mol Med. 2024. PMID: 38606593 Free PMC article.
-
Molecular Characterization of Collagen Hydroxylysine O-Glycosylation by Mass Spectrometry: Current Status.Aust J Chem. 2013 Jul 18;66(7):760-769. doi: 10.1071/CH13174. Aust J Chem. 2013. PMID: 25414518 Free PMC article.
-
Role of Glycosyltransferase 25 Domain 1 in Type I Collagen Glycosylation and Molecular Phenotypes.Biochemistry. 2019 Dec 17;58(50):5040-5051. doi: 10.1021/acs.biochem.8b00984. Epub 2019 Dec 5. Biochemistry. 2019. PMID: 31726007 Free PMC article.
References
-
- Marastoni S., Ligresti G., Lorenzon E., Colombatti A., Mongiat M. (2008) Connect. Tissue Res. 49, 203–206 - PubMed
-
- Rowe R. G., Weiss S. J. (2008) Trends Cell Biol. 18, 560–574 - PubMed
-
- Holmbeck K., Szabova L. (2006) Birth Defects Res. C Embryo. Today 78, 11–23 - PubMed
-
- Khoshnoodi J., Cartailler J. P., Alvares K., Veis A., Hudson B. G. (2006) J. Biol. Chem. 281, 38117–38121 - PubMed
-
- Prockop D. J., Kivirikko K. I. (1995) Annu. Rev. Biochem. 64, 403–434 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

