The auto-ubiquitylation of E3 ubiquitin-protein ligase Chfr at G2 phase is required for accumulation of polo-like kinase 1 and mitotic entry in mammalian cells
- PMID: 21768102
- PMCID: PMC3162422
- DOI: 10.1074/jbc.M111.231803
The auto-ubiquitylation of E3 ubiquitin-protein ligase Chfr at G2 phase is required for accumulation of polo-like kinase 1 and mitotic entry in mammalian cells
Abstract
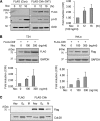
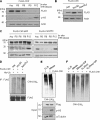
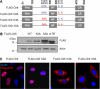
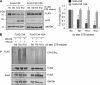
The E3 ubiquitin-protein ligase Chfr is a mitotic stress checkpoint protein that delays mitotic entry in response to microtubule damage; however, the molecular mechanism by which Chfr accomplishes this remains elusive. Here, we show that Chfr levels are elevated in response to microtubule-damaging stress. Moreover, G(2)/M transition is associated with cell cycle-dependent turnover of Chfr accompanied by high autoubiquitylation activity, suggesting that regulation of Chfr levels and auto-ubiquitylation activity are functionally significant. To test this, we generated Chfr mutants Chfr-K2A and Chfr-K5A in which putative lysine target sites of auto-ubiquitylation were replaced with alanine. Chfr-K2A did not undergo cell cycle-dependent degradation, and its levels remained high during G(2)/M phase. The elevated levels of Chfr-K2A caused a significant reduction in phosphohistone H3 levels and cyclinB1/Cdk1 kinase activities, leading to mitotic entry delay. Notably, polo-like kinase 1 levels at G(2) phase, but not at S phase, were ∼2-3-fold lower in cells expressing Chfr-K2A than in wild-type Chfr-expressing cells. Consistent with this, ubiquitylation of Plk1 at G(2) phase was accelerated in Chfr-K2A-expressing cells. In contrast, Aurora A levels remained constant, indicating that Plk1 is a major target of Chfr in controlling the timing of mitotic entry. Indeed, overexpression of Plk1 in Chfr-K2A-expressing cells restored cyclin B1/Cdk1 kinase activity and promoted mitotic entry. Collectively, these data indicate that Chfr auto-ubiquitylation is required to allow Plk1 to accumulate to levels necessary for activation of cyclin B1/Cdk1 kinase and mitotic entry. Our results provide the first evidence that Chfr auto-ubiquitylation and degradation are important for the G(2)/M transition.
Figures
Similar articles
-
The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition.J Cell Biol. 2002 Jan 21;156(2):249-59. doi: 10.1083/jcb.200108016. Epub 2002 Jan 21. J Cell Biol. 2002. PMID: 11807090 Free PMC article.
-
A Xenopus cell-free system for analysis of the Chfr ubiquitin ligase involved in control of mitotic entry.Methods Mol Biol. 2004;280:229-43. doi: 10.1385/1-59259-788-2:229. Methods Mol Biol. 2004. PMID: 15187257
-
FRET-Based Sorting of Live Cells Reveals Shifted Balance between PLK1 and CDK1 Activities During Checkpoint Recovery.Cells. 2020 Sep 19;9(9):2126. doi: 10.3390/cells9092126. Cells. 2020. PMID: 32961751 Free PMC article.
-
[Advance of study on effects of Chfr gene of mitosis prophase checkpoint--review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004 Dec;12(6):870-4. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004. PMID: 15631682 Review. Chinese.
-
Polo-like kinase 1: target and regulator of anaphase-promoting complex/cyclosome-dependent proteolysis.Cancer Res. 2006 Jul 15;66(14):6895-8. doi: 10.1158/0008-5472.CAN-06-0358. Cancer Res. 2006. PMID: 16849530 Review.
Cited by
-
CHFR: a key checkpoint component implicated in a wide range of cancers.Cell Mol Life Sci. 2012 May;69(10):1669-87. doi: 10.1007/s00018-011-0892-2. Epub 2011 Dec 13. Cell Mol Life Sci. 2012. PMID: 22159584 Free PMC article. Review.
-
Vaccinia-related kinase 2 drives pancreatic cancer progression by protecting Plk1 from Chfr-mediated degradation.Oncogene. 2021 Jul;40(28):4663-4674. doi: 10.1038/s41388-021-01893-4. Epub 2021 Jun 17. Oncogene. 2021. PMID: 34140642
-
Sustained expression of miR-26a promotes chromosomal instability and tumorigenesis through regulation of CHFR.Nucleic Acids Res. 2017 May 5;45(8):4401-4412. doi: 10.1093/nar/gkx022. Nucleic Acids Res. 2017. PMID: 28126920 Free PMC article.
-
Damaged replication forks tolerate USP7 to maintain genome stability.Mol Cell Oncol. 2015 Jul 6;3(1):e1063571. doi: 10.1080/23723556.2015.1063571. eCollection 2016 Jan. Mol Cell Oncol. 2015. PMID: 27308573 Free PMC article.
-
Emerging evidence for CHFR as a cancer biomarker: from tumor biology to precision medicine.Cancer Metastasis Rev. 2014 Mar;33(1):161-71. doi: 10.1007/s10555-013-9462-4. Cancer Metastasis Rev. 2014. PMID: 24375389 Free PMC article. Review.
References
-
- Rieder C. L., Cole R. (2000) Cold Spring Harbor Symp. Quant. Biol. 65, 369–376 - PubMed
-
- Scolnick D. M., Halazonetis T. D. (2000) Nature 406, 430–435 - PubMed
-
- Satoh A., Toyota M., Itoh F., Sasaki Y., Suzuki H., Ogi K., Kikuchi T., Mita H., Yamashita T., Kojima T., Kusano M., Fujita M., Hosokawa M., Endo T., Tokino T., Imai K. (2003) Cancer Res. 63, 8606–8613 - PubMed
-
- Banno K., Yanokura M., Kawaguchi M., Kuwabara Y., Akiyoshi J., Kobayashi Y., Iwata T., Hirasawa A., Fujii T., Susumu N., Tsukazaki K., Aoki D. (2007) Int. J. Oncol. 31, 713–720 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

