The F-BAR protein Rapostlin regulates dendritic spine formation in hippocampal neurons
- PMID: 21768103
- PMCID: PMC3173151
- DOI: 10.1074/jbc.M111.236265
The F-BAR protein Rapostlin regulates dendritic spine formation in hippocampal neurons
Abstract
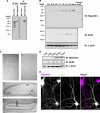
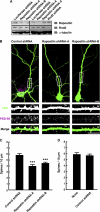
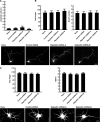
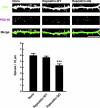
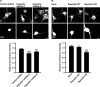
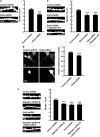
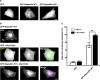
Pombe Cdc15 homology proteins, characterized by Fer/CIP4 homology Bin-Amphiphysin-Rvs/extended Fer/CIP4 homology (F-BAR/EFC) domains with membrane invaginating property, play critical roles in a variety of membrane reorganization processes. Among them, Rapostlin/formin-binding protein 17 (FBP17) has attracted increasing attention as a critical coordinator of endocytosis. Here we found that Rapostlin was expressed in the developing rat brain, including the hippocampus, in late developmental stages when accelerated dendritic spine formation and maturation occur. In primary cultured rat hippocampal neurons, knockdown of Rapostlin by shRNA or overexpression of Rapostlin-QQ, an F-BAR domain mutant of Rapostlin that has no ability to induce membrane invagination, led to a significant decrease in spine density. Expression of shRNA-resistant wild-type Rapostlin effectively restored spine density in Rapostlin knockdown neurons, whereas expression of Rapostlin deletion mutants lacking the protein kinase C-related kinase homology region 1 (HR1) or Src homology 3 (SH3) domain did not. In addition, knockdown of Rapostlin or overexpression of Rapostlin-QQ reduced the uptake of transferrin in hippocampal neurons. Knockdown of Rnd2, which binds to the HR1 domain of Rapostlin, also reduced spine density and the transferrin uptake. These results suggest that Rapostlin and Rnd2 cooperatively regulate spine density. Indeed, Rnd2 enhanced the Rapostlin-induced tubular membrane invagination. We conclude that the F-BAR protein Rapostlin, whose activity is regulated by Rnd2, plays a key role in spine formation through the regulation of membrane dynamics.
Figures

Similar articles
-
Rapostlin is a novel effector of Rnd2 GTPase inducing neurite branching.J Biol Chem. 2002 Nov 22;277(47):45428-34. doi: 10.1074/jbc.M208090200. Epub 2002 Sep 18. J Biol Chem. 2002. PMID: 12244061
-
Identification of splicing variants of Rapostlin, a novel RND2 effector that interacts with neural Wiskott-Aldrich syndrome protein and induces neurite branching.J Biol Chem. 2004 Apr 2;279(14):14104-10. doi: 10.1074/jbc.M312763200. Epub 2004 Jan 19. J Biol Chem. 2004. PMID: 14732713
-
Regulation of neuronal morphology by Toca-1, an F-BAR/EFC protein that induces plasma membrane invagination.J Biol Chem. 2006 Sep 29;281(39):29042-53. doi: 10.1074/jbc.M604025200. Epub 2006 Aug 2. J Biol Chem. 2006. PMID: 16885158
-
Different functional modes of BAR domain proteins in formation and plasticity of mammalian postsynapses.J Cell Sci. 2015 Sep 1;128(17):3177-85. doi: 10.1242/jcs.174193. Epub 2015 Aug 18. J Cell Sci. 2015. PMID: 26285709 Review.
-
F-BAR family proteins, emerging regulators for cell membrane dynamic changes-from structure to human diseases.J Hematol Oncol. 2015 May 9;8:47. doi: 10.1186/s13045-015-0144-2. J Hematol Oncol. 2015. PMID: 25956236 Free PMC article. Review.
Cited by
-
High expression of FBP17 in invasive breast cancer cells promotes invadopodia formation.Med Oncol. 2018 Apr 12;35(5):71. doi: 10.1007/s12032-018-1132-5. Med Oncol. 2018. PMID: 29651632
-
Luciferase shRNA Presents off-Target Effects on Voltage-Gated Ion Channels in Mouse Hippocampal Pyramidal Neurons.eNeuro. 2017 Oct 11;4(5):ENEURO.0186-17.2017. doi: 10.1523/ENEURO.0186-17.2017. eCollection 2017 Sep-Oct. eNeuro. 2017. PMID: 29034317 Free PMC article.
-
A genetic correlation and bivariate genome-wide association study of grip strength and depression.PLoS One. 2022 Dec 15;17(12):e0278392. doi: 10.1371/journal.pone.0278392. eCollection 2022. PLoS One. 2022. PMID: 36520780 Free PMC article.
-
Function and regulation of Rnd proteins in cortical projection neuron migration.Front Neurosci. 2015 Feb 6;9:19. doi: 10.3389/fnins.2015.00019. eCollection 2015. Front Neurosci. 2015. PMID: 25705175 Free PMC article. Review.
-
Mechanical Principles Governing the Shapes of Dendritic Spines.Front Physiol. 2021 Jun 16;12:657074. doi: 10.3389/fphys.2021.657074. eCollection 2021. Front Physiol. 2021. PMID: 34220531 Free PMC article.
References
-
- Hall A. (1998) Science 279, 509–514 - PubMed
-
- Luo L. (2000) Nat. Rev. Neurosci. 1, 173–180 - PubMed
-
- Negishi M., Katoh H. (2002) J. Biochem. 132, 157–166 - PubMed
-
- Burridge K., Wennerberg K. (2004) Cell 116, 167–179 - PubMed
-
- Aspenström P., Fransson A., Richnau N. (2006) Trends Biochem. Sci. 31, 670–679 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

