The comparative ecology and biogeography of parasites
- PMID: 21768153
- PMCID: PMC3130428
- DOI: 10.1098/rstb.2011.0048
The comparative ecology and biogeography of parasites
Abstract
Comparative ecology uses interspecific relationships among traits, while accounting for the phylogenetic non-independence of species, to uncover general evolutionary processes. Applied to biogeographic questions, it can be a powerful tool to explain the spatial distribution of organisms. Here, we review how comparative methods can elucidate biogeographic patterns and processes, using analyses of distributional data on parasites (fleas and helminths) as case studies. Methods exist to detect phylogenetic signals, i.e. the degree of phylogenetic dependence of a given character, and either to control for these signals in statistical analyses of interspecific data, or to measure their contribution to variance. Parasite-host interactions present a special case, as a given trait may be a parasite trait, a host trait or a property of the coevolved association rather than of one participant only. For some analyses, it is therefore necessary to correct simultaneously for both parasite phylogeny and host phylogeny, or to evaluate which has the greatest influence on trait expression. Using comparative approaches, we show that two fundamental properties of parasites, their niche breadth, i.e. host specificity, and the nature of their life cycle, can explain interspecific and latitudinal variation in the sizes of their geographical ranges, or rates of distance decay in the similarity of parasite communities. These findings illustrate the ways in which phylogenetically based comparative methods can contribute to biogeographic research.
Figures
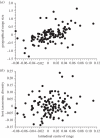
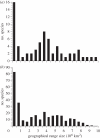
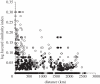
References
-
- McIntosh R. 1985. The background of ecology: concept and theory. New York, NY: Cambridge University Press
-
- Brooks D. R., McLennan D. A. 1991. Phylogeny, ecology, and behavior. Chicago, IL: University of Chicago Press
-
- Schoener T. W. 1965. The evolution of bill size differences among sympatric congeneric species of birds. Evolution 19, 189–21310.2307/2406374 (doi:10.2307/2406374) - DOI - DOI
-
- Schoener T. W., Gorman G. C. 1968. Some niche differences in three Lesser Antillean lizards of the genus Anolis. Ecology 49, 819–83010.2307/1936533 (doi:10.2307/1936533) - DOI - DOI
-
- Roughgarden J. 1974. Niche width: biogeographic patterns among Anolis lizard populations. Am. Nat. 108, 429–44210.1086/282924 (doi:10.1086/282924) - DOI - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
