HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore
- PMID: 21768289
- PMCID: PMC3144403
- DOI: 10.1083/jcb.201012017
HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore
Abstract
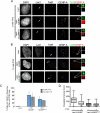
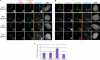
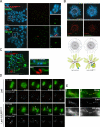
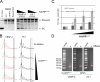
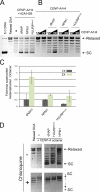
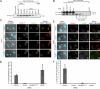
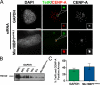
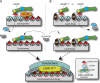
Centromeres of higher eukaryotes are epigenetically marked by the centromere-specific CENP-A nucleosome. New CENP-A recruitment requires the CENP-A histone chaperone HJURP. In this paper, we show that a LacI (Lac repressor) fusion of HJURP drove the stable recruitment of CENP-A to a LacO (Lac operon) array at a noncentromeric locus. Ectopically targeted CENP-A chromatin at the LacO array was sufficient to direct the assembly of a functional centromere as indicated by the recruitment of the constitutive centromere-associated network proteins, the microtubule-binding protein NDC80, and the formation of stable kinetochore-microtubule attachments. An amino-terminal fragment of HJURP was able to assemble CENP-A nucleosomes in vitro, demonstrating that HJURP is a chromatin assembly factor. Furthermore, HJURP recruitment to endogenous centromeres required the Mis18 complex. Together, these data suggest that the role of the Mis18 complex in CENP-A deposition is to recruit HJURP and that the CENP-A nucleosome assembly activity of HJURP is responsible for centromeric chromatin assembly to maintain the epigenetic mark.
Figures
References
-
- Bergmann J.H., Rodríguez M.G., Martins N.M., Kimura H., Kelly D.A., Masumoto H., Larionov V., Jansen L.E., Earnshaw W.C. 2011. Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. EMBO J. 30:328–340 10.1038/emboj.2010.329 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

