A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax
- PMID: 21768300
- PMCID: PMC3438884
- DOI: 10.1182/blood-2011-04-348748
A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax
Abstract
Currently, there are no reliable RBC invasion assays to guide the discovery of vaccines against Plasmodium vivax, the most prevalent malaria parasite in Asia and South America. Here we describe a protocol for an ex vivo P vivax invasion assay that can be easily deployed in laboratories located in endemic countries. The assay is based on mixing enriched cord blood reticulocytes with matured, trypsin-treated P vivax schizonts concentrated from clinical isolates. The reliability of this assay was demonstrated using a large panel of P vivax isolates freshly collected from patients in Thailand.
Figures
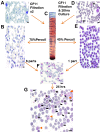





Comment in
-
"Baby" red cells to the rescue.Blood. 2011 Sep 29;118(13):3454-5. doi: 10.1182/blood-2011-08-370395. Blood. 2011. PMID: 21960678 No abstract available.
References
-
- Baird JK. Neglect of Plasmodium vivax malaria. Trends Parasitol. 2007;23(11):533–539. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

