Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose
- PMID: 21768335
- PMCID: PMC3150898
- DOI: 10.1073/pnas.1108455108
Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose
Abstract
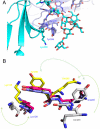
Antibody-mediated cellular cytotoxicity (ADCC), a key immune effector mechanism, relies on the binding of antigen-antibody complexes to Fcγ receptors expressed on immune cells. Antibodies lacking core fucosylation show a large increase in affinity for FcγRIIIa leading to an improved receptor-mediated effector function. Although afucosylated IgGs exist naturally, a next generation of recombinant therapeutic, glycoenginereed antibodies is currently being developed to exploit this finding. In this study, the crystal structures of a glycosylated Fcγ receptor complexed with either afucosylated or fucosylated Fc were determined allowing a detailed, molecular understanding of the regulatory role of Fc-oligosaccharide core fucosylation in improving ADCC. The structures reveal a unique type of interface consisting of carbohydrate-carbohydrate interactions between glycans of the receptor and the afucosylated Fc. In contrast, in the complex structure with fucosylated Fc, these contacts are weakened or nonexistent, explaining the decreased affinity for the receptor. These findings allow us to understand the higher efficacy of therapeutic antibodies lacking the core fucose and also suggest a unique mechanism by which the immune system can regulate antibody-mediated effector functions.
Conflict of interest statement
Conflict of interest statement: The authors are employed by F. Hoffmann–La Roche AG and Roche Glycart AG.
Figures



References
-
- Jefferis R. Recombinant antibody therapeutics: The impact of glycosylation on mechanisms of action. Trends Pharmacol Sci. 2009;30:356–362. - PubMed
-
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. - PubMed
-
- Jefferis R, Lund J. Interaction sites on human IgG-Fc for FcgammaR: Current models. Immunol Lett. 2002;82:57–65. - PubMed
-
- Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406:267–273. - PubMed
-
- Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 2003;325:979–989. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

