Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome
- PMID: 21768372
- PMCID: PMC3150921
- DOI: 10.1073/pnas.1006437108
Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome
Abstract
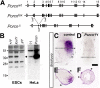
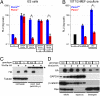
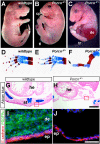
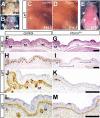
The Drosophila porcupine gene is required for secretion of wingless and other Wnt proteins, and sporadic mutations in its unique human ortholog, PORCN, cause a pleiotropic X-linked dominant disorder, focal dermal hypoplasia (FDH, also known as Goltz syndrome). We generated a conditional allele of the X-linked mouse Porcn gene and analyzed its requirement in Wnt signaling and embryonic development. We find that Porcn-deficient cells exhibit a cell-autonomous defect in Wnt ligand secretion but remain responsive to exogenous Wnts. Consistent with the female-specific inheritance pattern of FDH, Porcn hemizygous male embryos arrest during early embryogenesis and fail to generate mesoderm, a phenotype previously associated with loss of Wnt activity. Heterozygous Porcn mutant females exhibit a spectrum of limb, skin, and body patterning abnormalities resembling those observed in human patients with FDH. Many of these defects are recapitulated by ectoderm-specific deletion of Porcn, substantiating a long-standing hypothesis regarding the etiology of human FDH and extending previous studies that have focused on downstream elements of Wnt signaling, such as β-catenin. Conditional deletion of Porcn thus provides an experimental model of FDH, as well as a valuable tool to probe Wnt ligand function in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

