G protein G(alpha)o is essential for vomeronasal function and aggressive behavior in mice
- PMID: 21768373
- PMCID: PMC3150917
- DOI: 10.1073/pnas.1107770108
G protein G(alpha)o is essential for vomeronasal function and aggressive behavior in mice
Abstract
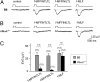
The rodent vomeronasal organ (VNO) mediates the regulation of species-specific and interspecies social behaviors. We have used gene targeting to examine the role of the G protein Gαo, encoded by the gene Gnao1, in vomeronasal function. We used the Cre-loxP system to delete Gαo in those cells that express olfactory marker protein, which includes all vomeronasal sensory neurons of the basal layer of the VNO sensory epithelium. Using electrophysiology and calcium imaging, we show that the conditional null mice exhibit strikingly reduced sensory responses in V2R receptor-expressing vomeronasal sensory neurons to specific molecular cues, including MHC1 antigens, major urinary proteins, and exocrine gland-secreting peptide. Gαo is also vital for vomeronasal sensing of two N-formylated mitochondrially encoded peptides derived from NADH dehydrogenase 1. Furthermore, we show that Gαo is an essential requirement for the display of male-male territorial aggression as well as maternal aggression in mice. Finally, we show that Gαo-dependent maternal aggression can be induced by major urinary proteins. These cellular and behavioral phenotypes identify Gαo as the primary G-protein α-subunit mediating the detection of peptide and protein pheromones by sensory neurons of the VNO.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

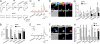
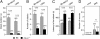

References
-
- Munger SD, Leinders-Zufall T, Zufall F. Subsystem organization of the mammalian sense of smell. Annu Rev Physiol. 2009;71:115–140. - PubMed
-
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. - PubMed
-
- Tirindelli R, Dibattista M, Pifferi S, Menini A. From pheromones to behavior. Physiol Rev. 2009;89:921–956. - PubMed
-
- Jia C, Halpern M. Subclasses of vomeronasal receptor neurons: Differential expression of G proteins (Giα2 and Goα) and segregated projections to the accessory olfactory bulb. Brain Res. 1996;719:117–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

