G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination
- PMID: 21768377
- PMCID: PMC3150909
- DOI: 10.1073/pnas.1104821108
G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination
Abstract
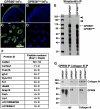
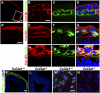
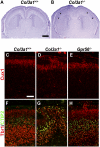
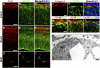
GPR56, an orphan G protein-coupled receptor (GPCR) from the family of adhesion GPCRs, plays an indispensable role in cortical development and lamination. Mutations in the GPR56 gene cause a malformed cerebral cortex in both humans and mice that resembles cobblestone lissencephaly, which is characterized by overmigration of neurons beyond the pial basement membrane. However, the molecular mechanisms through which GPR56 regulates cortical development remain elusive due to the unknown status of its ligand. Here we identify collagen, type III, alpha-1 (gene symbol Col3a1) as the ligand of GPR56 through an in vitro biotinylation/proteomics approach. Further studies demonstrated that Col3a1 null mutant mice exhibit overmigration of neurons beyond the pial basement membrane and a cobblestone-like cortical malformation similar to the phenotype seen in Gpr56 null mutant mice. Functional studies suggest that the interaction of collagen III with its receptor GPR56 inhibits neural migration in vitro. As for intracellular signaling, GPR56 couples to the Gα(12/13) family of G proteins and activates RhoA pathway upon ligand binding. Thus, collagen III regulates the proper lamination of the cerebral cortex by acting as the major ligand of GPR56 in the developing brain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
GPR56 functions together with α3β1 integrin in regulating cerebral cortical development.PLoS One. 2013 Jul 9;8(7):e68781. doi: 10.1371/journal.pone.0068781. Print 2013. PLoS One. 2013. PMID: 23874761 Free PMC article.
-
Bi-allelic variants in COL3A1 encoding the ligand to GPR56 are associated with cobblestone-like cortical malformation, white matter changes and cerebellar cysts.J Med Genet. 2017 Jun;54(6):432-440. doi: 10.1136/jmedgenet-2016-104421. Epub 2017 Mar 3. J Med Genet. 2017. PMID: 28258187
-
Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a G alpha 12/13 and Rho pathway.J Biol Chem. 2008 May 23;283(21):14469-78. doi: 10.1074/jbc.M708919200. Epub 2008 Mar 31. J Biol Chem. 2008. PMID: 18378689
-
GPR56 and the developing cerebral cortex: cells, matrix, and neuronal migration.Mol Neurobiol. 2013 Feb;47(1):186-96. doi: 10.1007/s12035-012-8343-0. Epub 2012 Sep 22. Mol Neurobiol. 2013. PMID: 23001883 Free PMC article. Review.
-
Adhesion-GPCRs in the CNS.Adv Exp Med Biol. 2010;706:87-97. doi: 10.1007/978-1-4419-7913-1_7. Adv Exp Med Biol. 2010. PMID: 21618828 Review.
Cited by
-
Malformations of cortical development and epilepsy.Cold Spring Harb Perspect Med. 2015 May 1;5(5):a022392. doi: 10.1101/cshperspect.a022392. Cold Spring Harb Perspect Med. 2015. PMID: 25934463 Free PMC article. Review.
-
G Protein-Coupled Receptors in Myelinating Glia.Trends Pharmacol Sci. 2016 Nov;37(11):977-987. doi: 10.1016/j.tips.2016.09.002. Epub 2016 Sep 23. Trends Pharmacol Sci. 2016. PMID: 27670389 Free PMC article. Review.
-
International consensus recommendations on the diagnostic work-up for malformations of cortical development.Nat Rev Neurol. 2020 Nov;16(11):618-635. doi: 10.1038/s41582-020-0395-6. Epub 2020 Sep 7. Nat Rev Neurol. 2020. PMID: 32895508 Free PMC article. Review.
-
Spatiotemporal Regulation of Rho GTPases in Neuronal Migration.Cells. 2019 Jun 10;8(6):568. doi: 10.3390/cells8060568. Cells. 2019. PMID: 31185627 Free PMC article. Review.
-
The role of GPR56/ADGRG1 in health and disease.Biomed J. 2021 Oct;44(5):534-547. doi: 10.1016/j.bj.2021.04.012. Epub 2021 May 4. Biomed J. 2021. PMID: 34654683 Free PMC article. Review.
References
-
- Gaitanis JN, Walsh CA. Genetics of disorders of cortical development. Neuroimaging Clin N Am. 2004;14:219–229. viii. - PubMed
-
- Ayala R, Shu T, Tsai LH. Trekking across the brain: The journey of neuronal migration. Cell. 2007;128:29–43. - PubMed
-
- Marín O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. - PubMed
-
- Caviness VS, Jr, Rakic P. Mechanisms of cortical development: A view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. - PubMed
-
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

