Response to antimalarial agents in cutaneous lupus erythematosus: a prospective analysis
- PMID: 21768444
- PMCID: PMC3282057
- DOI: 10.1001/archdermatol.2011.191
Response to antimalarial agents in cutaneous lupus erythematosus: a prospective analysis
Abstract
Objective: To demonstrate response to antimalarial agents in patients with cutaneous lupus erythematosus (CLE) using activity scores from the Cutaneous Lupus Erythematosus Disease Area and Severity Index, a validated outcome measure.
Design: Prospective, longitudinal cohort study.
Setting: University cutaneous autoimmune disease clinic.
Participants: A total of 128 patients with CLE who presented from January 2007 to July 2010 and had at least 2 visits with activity scores.
Intervention: Administration of antimalarial agents.
Main outcome measures: Response was defined by a 4-point or 20% decrease in activity score. Response to initiation was determined by the difference between the scores before treatment and at the first visit at least 2 months after treatment. Response to continuation was determined by the difference between the scores at the first visit and the most recent visit while undergoing treatment.
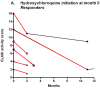
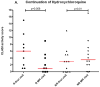
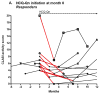
Results: Of 11 patients who initiated treatment with hydroxychloroquine, 55% were responders (n = 6), showing a decrease in median (interquartile range [IQR]) activity score from 8.0 (3.5-13.0) to 3.0 (1.8-7.3) (P = .03). Of 15 patients for whom hydroxychloroquine failed, 67% were responders to initiation of hydroxychloroquine-quinacrine therapy (n = 10), showing a decrease in median (IQR) activity score from 6.0 (4.8-8.3) to 3.0 (0.75-5.0) (P = .004). Nine of 21 patients who continued hydroxychloroquine treatment (43%), and 9 of 21 patients who continued hydroxychloroquine-quinacrine (43%) were responders, showing a decrease in median (IQR) activity score from 6.0 (1.5-9.5) to 1.0 (0.0-4.5) (P = .01) and 8.5 (4.25-17.5) to 5.0 (0.5-11.5) (P = .01), respectively.
Conclusions: The use of quinacrine with hydroxychloroquine is associated with response in patients for whom hydroxychloroquine monotherapy fails. Further reduction in disease activity can be associated with continuation of treatment with antimalarial agents.
Figures



Comment in
-
Practice Gaps. Optimizing antimalarial therapy for cutaneous lupus erythematosus: comment on "Response to antimalarial agents in cutaneous lupus erythematosus".Arch Dermatol. 2011 Nov;147(11):1267-8. doi: 10.1001/archdermatol.2011.336. Arch Dermatol. 2011. PMID: 22106112 No abstract available.
References
-
- Payne J. A postgraduate lecture on lupus erythematosus. Clin J. 1894;4:223–229.
-
- Office of Surgeon General. Circular letter N 153. The drug treatment of malaria, suppressive and clinical. JAMA. 1943;123:205–208.
-
- Page F. Treatment of lupus erythematosus with mepacrine. Lancet. 1951 Oct 27;2(6687):755–758. - PubMed
-
- Isaacson D, Elgart M, Turner ML. Anti-malarials in dermatology. Int J Dermatol. 1982 Sep;21(7):379–395. - PubMed
-
- Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: Update of therapeutic options Part I and Part II. J Am Acad Dermatol. 2010 Aug 23; - PubMed

