Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31
- PMID: 21768458
- PMCID: PMC3164242
- DOI: 10.1200/JCO.2011.35.0868
Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31
Abstract
Purpose: Trastuzumab is a humanized monoclonal antibody against the human epidermal growth factor receptor 2 (HER2). The clinical benefits of adjuvant trastuzumab have been demonstrated in interim analyses of four large trials. Initial data of the combined analysis of the North Central Cancer Treatment Group (NCCTG) N9831 Intergroup trial and National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trial were reported in 2005. Long-term follow-up results on disease-free survival (DFS) and overall survival (OS) have been awaited.
Patients and methods: Patients with HER2-positive operable breast cancer were randomly assigned to doxorubicin plus cyclophosphamide followed by paclitaxel with or without trastuzumab in the NCCTG N9831 and NSABP B-31 trials. The similar design of both trials allowed data from the control and trastuzumab-containing arms to be combined in a joint analysis.
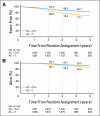
Results: At 3.9 years of median follow-up, there continues to be a highly statistically significant reduction in DFS event rate in favor of the trastuzumab-containing arm (P < .001). Similarly, there continues to be a statistically significant 39% reduction in death rate in favor of the trastuzumab-containing arm (P < .001).
Conclusion: These data demonstrate consistent DFS and OS advantages of adjuvant trastuzumab over time, with the longest follow-up reported to date. The clinical benefits continue to outweigh the risks of adverse effects.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures




References
-
- Genentech. Herceptin (trastuzumab). Package insert. 2009. Mar, http://www.gene.com/gene/products/information/pdf/herceptin-prescribing.pdf.
-
- Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. - PubMed
-
- Sjögren S, Inganas M, Lindgren A, et al. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–469. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

