High-dose chemotherapy with autologous stem-cell support as adjuvant therapy in breast cancer: overview of 15 randomized trials
- PMID: 21768471
- PMCID: PMC4322115
- DOI: 10.1200/JCO.2010.32.5910
High-dose chemotherapy with autologous stem-cell support as adjuvant therapy in breast cancer: overview of 15 randomized trials
Abstract
Purpose: Adjuvant high-dose chemotherapy (HDC) with autologous hematopoietic stem-cell transplantation (AHST) for high-risk primary breast cancer has not been shown to prolong survival. Individual trials have had limited power to show overall benefit or benefits within subsets.
Methods: We assembled individual patient data from 15 randomized trials that compared HDC versus control therapy without stem-cell support. Prospectively defined primary end points were relapse-free survival (RFS) and overall survival (OS). We compared the effect of HDC versus control by using log-rank tests and proportional hazards regression, and we adjusted for clinically relevant covariates. Subset analyses were by age, number of positive lymph nodes, tumor size, histology, hormone receptor (HmR) status, and human epidermal growth factor receptor 2 (HER2) status.
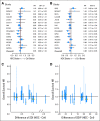
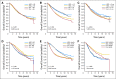
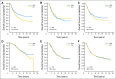
Results: Of 6,210 total patients (n = 3,118, HDC; n = 3,092 control), the median age was 46 years; 69% were premenopausal, 29% were postmenopausal, and 2% were unknown menopausal status; 49.5% were HmR positive; 33.5% were HmR negative, and 17% were unknown HmR status. The median follow-up was 6 years. After analysis was adjusted for covariates, HDC was found to prolong relapse-free survival (RFS; hazard ratio [HR], 0.87; 95% CI, 0.81 to 0.93; P < .001) but not overall survival (OS; HR, 0.94; 95% CI, 0.87 to 1.02; P = .13). For OS, no covariates had statistically significant interactions with treatment effect, and no subsets evinced a significant effect of HDC. Younger patients had a significantly better RFS on HDC than did older patients.
Conclusion: Adjuvant HDC with AHST prolonged RFS in high-risk primary breast cancer compared with control, but this did not translate into a significant OS benefit. Whether HDC benefits patients in the context of targeted therapies is unknown.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
The era of high-dose chemotherapy for breast cancer: revisiting a troubled quest.J Clin Oncol. 2011 Aug 20;29(24):3205-6. doi: 10.1200/JCO.2011.36.1303. Epub 2011 Jul 18. J Clin Oncol. 2011. PMID: 21768457 No abstract available.
-
High-dose chemotherapy as adjuvant therapy in breast cancer: could there be a survival benefit?J Clin Oncol. 2012 Mar 1;30(7):759; author reply 759-60. doi: 10.1200/JCO.2011.39.8057. Epub 2011 Dec 27. J Clin Oncol. 2012. PMID: 22203770 No abstract available.
-
Dose intensification in hormone receptor-negative and/or human epidermal growth factor receptor 2-negative high-risk primary breast cancer.J Clin Oncol. 2012 Mar 1;30(7):758; author reply 759-60. doi: 10.1200/JCO.2011.39.7257. Epub 2011 Dec 27. J Clin Oncol. 2012. PMID: 22203774 No abstract available.
References
-
- National Cancer Institute. Washington, DC: National Cancer Institute; 2001. High-Dose Chemotherapy for Breast Cancer: History. http://www.cancer.gov/clinicaltrials/developments/high-dose-chemo-histor....
-
- Antman KH. New developments in clinical oncology: The interdependence of bench and bedside. Cancer Res. 1991;51(suppl 18):5060s–5064s. - PubMed
-
- Fisher B, Anderson S, Wickerham DL, et al. Increased intensification and total dose of cyclophosphamide in a doxorubicin-cyclophosphamide regimen for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-22. J Clin Oncol. 1997;15:1858–1869. - PubMed
-
- Fisher B, Anderson S, DeCillis A, et al. Further evaluation of intensified and increased total dose of cyclophosphamide for the treatment of primary breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-25. J Clin Oncol. 1999;17:3374–3388. - PubMed
-
- Henderson IC, Berry DA, Demetri GD. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

