Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis
- PMID: 21768580
- PMCID: PMC3793257
- DOI: 10.7326/0003-4819-155-2-201107190-00002
Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis
Abstract
Background: Testing has been advocated for all persons with newly diagnosed colorectal cancer to identify families with the Lynch syndrome, an autosomal dominant cancer-predisposition syndrome that is a paradigm for personalized medicine.
Objective: To estimate the effectiveness and cost-effectiveness of strategies to identify the Lynch syndrome, with attention to sex, age at screening, and differential effects for probands and relatives.
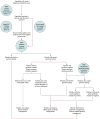
Design: Markov model that incorporated risk for colorectal, endometrial, and ovarian cancers.
Data sources: Published literature.
Target population: All persons with newly diagnosed colorectal cancer and their relatives.
Time horizon: Lifetime.
Perspective: Third-party payer.
Intervention: Strategies based on clinical criteria, prediction algorithms, tumor testing, or up-front germline mutation testing, followed by tailored screening and risk-reducing surgery.
Outcome measures: Life-years, cancer cases and deaths, costs, and incremental cost-effectiveness ratios.
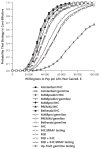
Results of base-case analysis: The benefit of all strategies accrued primarily to relatives with a mutation associated with the Lynch syndrome, particularly women, whose life expectancy could increase by approximately 4 years with hysterectomy and salpingo-oophorectomy and adherence to colorectal cancer screening recommendations. At current rates of germline testing, screening, and prophylactic surgery, the strategies reduced deaths from colorectal cancer by 7% to 42% and deaths from endometrial and ovarian cancer by 1% to 6%. Among tumor-testing strategies, immunohistochemistry followed by BRAF mutation testing was preferred, with an incremental cost-effectiveness ratio of $36,200 per life-year gained.
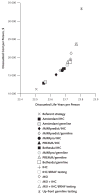
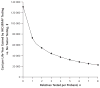
Results of sensitivity analysis: The number of relatives tested per proband was a critical determinant of both effectiveness and cost-effectiveness, with testing of 3 to 4 relatives required for most strategies to meet a threshold of $50,000 per life-year gained. Immunohistochemistry followed by BRAF mutation testing was preferred in 59% of iterations in probabilistic sensitivity analysis at a threshold of $100,000 per life-year gained. Screening for the Lynch syndrome with immunohistochemistry followed by BRAF mutation testing only up to age 70 years cost $44,000 per incremental life-year gained compared with screening only up to age 60 years, and screening without an upper age limit cost $88,700 per incremental life-year gained compared with screening only up to age 70 years.
Limitation: Other types of cancer, uncertain family pedigrees, and genetic variants of unknown significance were not considered.
Conclusion: Widespread colorectal tumor testing to identify families with the Lynch syndrome could yield substantial benefits at acceptable costs, particularly for women with a mutation associated with the Lynch syndrome who begin regular screening and have risk-reducing surgery. The cost-effectiveness of such testing depends on the participation rate among relatives at risk for the Lynch syndrome.
Primary funding source: National Institutes of Health.
Conflict of interest statement
Figures
Comment in
-
Who should have genetic testing for the Lynch syndrome?Ann Intern Med. 2011 Jul 19;155(2):127-8. doi: 10.7326/0003-4819-155-2-201107190-00009. Ann Intern Med. 2011. PMID: 21768586 No abstract available.
Summary for patients in
-
Summaries for patients. Comparing the benefits and costs of testing for genetic causes of colon cancer.Ann Intern Med. 2011 Jul 19;155(2):I36. doi: 10.7326/0003-4819-155-2-201107190-00001. Ann Intern Med. 2011. PMID: 21768567 No abstract available.
References
-
- Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer. 1999;81:214–8. - PubMed
-
- Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet. 1997;6:105–10. - PubMed
-
- Hendriks YM, de Jong AE, Morreau H, Tops CM, Vasen HF, Wijnen JT, et al. Diagnostic approach and management of Lynch syndrome (hereditary non-polyposis colorectal carcinoma): a guide for clinicians. CA Cancer J Clin. 2006;56:213–25. - PubMed
-
- Lynch HT, Boland CR, Gong G, Shaw TG, Lynch PM, Fodde R, et al. Phenotypic and genotypic heterogeneity in the Lynch syndrome: diagnostic, surveillance and management implications. Eur J Hum Genet. 2006;14:390–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
