Understanding the metabolic basis of drug resistance: therapeutic induction of the Warburg effect kills cancer cells
- PMID: 21768775
- PMCID: PMC3180190
- DOI: 10.4161/cc.10.15.16584
Understanding the metabolic basis of drug resistance: therapeutic induction of the Warburg effect kills cancer cells
Abstract
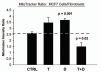
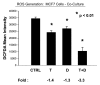
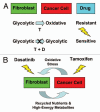
Previously, we identified a form of epithelial-stromal metabolic coupling, in which cancer cells induce aerobic glycolysis in adjacent stromal fibroblasts, via oxidative stress, driving autophagy and mitophagy. In turn, these cancer-associated fibroblasts provide recycled nutrients to epithelial cancer cells, "fueling" oxidative mitochondrial metabolism and anabolic growth. An additional consequence is that these glycolytic fibroblasts protect cancer cells against apoptosis, by providing a steady nutrient stream of to mitochondria in cancer cells. Here, we investigated whether these interactions might be the basis of tamoxifen-resistance in ER(+) breast cancer cells. We show that MCF7 cells alone are Tamoxifen-sensitive, but become resistant when co-cultured with hTERT-immortalized human fibroblasts. Next, we searched for a drug combination (Tamoxifen + Dasatinib) that could over-come fibroblast-induced Tamoxifen-resistance. Importantly, we show that this drug combination acutely induces the Warburg effect (aerobic glycolysis) in MCF7 cancer cells, abruptly cutting off their ability to use their fuel supply, effectively killing these cancer cells. Thus, we believe that the Warburg effect in tumor cells is not the "root cause" of cancer, but rather it may provide the necessary clues to preventing chemo-resistance in cancer cells. Finally, we observed that this drug combination (Tamoxifen + Dasatinib) also had a generalized anti-oxidant effect, on both co-cultured fibroblasts and cancer cells alike, potentially reducing tumor-stroma co-evolution. Our results are consistent with the idea that chemo-resistance may be both a metabolic and stromal phenomenon that can be overcome by targeting mitochondrial function in epithelial cancer cells. Thus, simultaneously targeting both (1) the tumor stroma and (2) the epithelial cancer cells, with combination therapies, may be the most successful approach to anti-cancer therapy. This general strategy of combination therapy for overcoming drug resistance could be applicable to many different types of cancer.
Figures




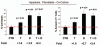





References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA075503/CA/NCI NIH HHS/United States
- R01 CA098779/CA/NCI NIH HHS/United States
- R01-CA-120876/CA/NCI NIH HHS/United States
- R01 CA120876/CA/NCI NIH HHS/United States
- R01-CA-70896/CA/NCI NIH HHS/United States
- R01-CA-098779/CA/NCI NIH HHS/United States
- R01-CA-86072/CA/NCI NIH HHS/United States
- R01-AR-055660/AR/NIAMS NIH HHS/United States
- R01-CA-080250/CA/NCI NIH HHS/United States
- R01 CA070896/CA/NCI NIH HHS/United States
- R01 CA107382/CA/NCI NIH HHS/United States
- P30 CA056036/CA/NCI NIH HHS/United States
- P30-CA-56036/CA/NCI NIH HHS/United States
- R01-CA-107382/CA/NCI NIH HHS/United States
- R01 AR055660/AR/NIAMS NIH HHS/United States
- R01-CA-75503/CA/NCI NIH HHS/United States
- R01 CA080250/CA/NCI NIH HHS/United States
- R01 CA086072/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
