Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors
- PMID: 21769103
- PMCID: PMC3197954
- DOI: 10.1038/leu.2011.185
Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors
Abstract
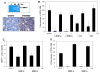
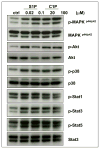
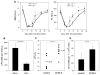
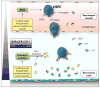
We have observed that conditioning for hematopoietic transplantation by lethal irradiation induces a proteolytic microenvironment in the bone marrow (BM) that activates the complement cascade (CC). As a result, BM is enriched for proteolytic enzymes and the soluble form of the terminal product of CC activation, the membrane attack complex C5b-C9 (MAC). At the same time, proteolytic enzymes induced in irradiated BM impair the chemotactic activity of α-chemokine stromal-derived factor-1 (SDF-1). As SDF-1 is considered a crucial BM chemoattractant for transplanted hematopoietic stem/progenitor cells (HSPCs), we sought to determine whether other factors that are resistant to proteolytic enzymes have a role in this process, focusing on proteolysis-resistant bioactive lipids. We found that the concentrations of sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P) increase in the BM after conditioning for transplantation and that both S1P and, as we show here for the first time, C1P are potent chemoattractants for HSPCs. Next, we observed that C5-deficient mice that do not generate MAC show impaired engraftment of HSPCs. In support of a role for MAC in homing and engraftment, we found that soluble MAC enhances in a CR3 (CD11b/CD18)-dependent manner the adhesion of HSPCs to BM stromal cells and increases the secretion of SDF-1 by BM stroma. We conclude that an increase in BM levels of proteolytic enzyme-resistant S1P and C1P and activation of CC, which leads to the generation of MAC, has an important and previously underappreciated role in the homing of transplanted HSPCs.
Figures




References
-
- Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. - PubMed
-
- Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, et al. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18:1482–1490. - PubMed
-
- Cho SY, Xu M, Roboz J, Lu M, Mascarenhas J, Hoffman R. The effect of CXCL12 processing on CD34+ cell migration in myeloproliferative neoplasms. Cancer Res. 2010;70:3402–3410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

