Enhanced sensitivity to cisplatin and gemcitabine in Brca1-deficient murine mammary epithelial cells
- PMID: 21771338
- PMCID: PMC3146825
- DOI: 10.1186/1471-2210-11-7
Enhanced sensitivity to cisplatin and gemcitabine in Brca1-deficient murine mammary epithelial cells
Abstract
Background: Breast cancers due to germline mutations or altered expression of the BRCA1 gene associate with an aggressive clinical course and frequently exhibit a "triple-negative" phenotype, i.e. lack of expression of the estrogen and progesterone hormone receptors and lack of overexpression of the HER2/NEU oncogene, thereby rendering them relatively insensitive to hormonal manipulation and targeted HER2 therapy, respectively. BRCA1 plays a role in multiple DNA repair pathways, and thus, when mutated, results in sensitivity to certain DNA damaging drugs.
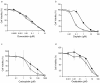
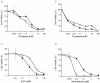
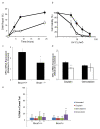
Results: Here, we used a Brca1 murine mammary epithelial cell (MMEC) model to examine the effect of loss of Brca1 on cellular sensitivity to various chemotherapy drugs. To explore novel therapeutic strategies, we included DNA damaging and non-DNA damaging drugs whose mechanisms are dependent and independent of DNA repair, respectively, and drugs that are used in standard and non-standard lines of therapy for breast cancer. To understand the cellular mechanism, we also determined the role that DNA repair plays in sensitivity to these drugs. We found that cisplatin and gemcitabine had the greatest specific therapeutic benefit to Brca1-deficient MMECs, and that when used in combination produced a synergistic effect. This sensitivity may be attributed in part to defective NER, which is one of the DNA repair pathways normally responsible for repairing DNA adducts produced by cisplatin and is shown in this study to be defective in Brca1-deficient MMECs. Brca1-deficient MMECs were not differentially sensitive to the standard breast cancer chemotherapy drugs doxorubicin, docetaxel or 5-FU.
Conclusions: Both cisplatin and gemcitabine should be explored in clinical trials for first line regimens for BRCA1-associated and triple-negative breast cancer.
Figures

References
-
- Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A. et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. doi: 10.1086/375033. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

