Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation
- PMID: 21771582
- PMCID: PMC3229836
- DOI: 10.1016/j.bbabio.2011.07.001
Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation
Abstract
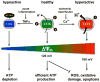
Cytochrome c (Cytc) and cytochrome c oxidase (COX) catalyze the terminal reaction of the mitochondrial electron transport chain (ETC), the reduction of oxygen to water. This irreversible step is highly regulated, as indicated by the presence of tissue-specific and developmentally expressed isoforms, allosteric regulation, and reversible phosphorylations, which are found in both Cytc and COX. The crucial role of the ETC in health and disease is obvious since it, together with ATP synthase, provides the vast majority of cellular energy, which drives all cellular processes. However, under conditions of stress, the ETC generates reactive oxygen species (ROS), which cause cell damage and trigger death processes. We here discuss current knowledge of the regulation of Cytc and COX with a focus on cell signaling pathways, including cAMP/protein kinase A and tyrosine kinase signaling. Based on the crystal structures we highlight all identified phosphorylation sites on Cytc and COX, and we present a new phosphorylation site, Ser126 on COX subunit II. We conclude with a model that links cell signaling with the phosphorylation state of Cytc and COX. This in turn regulates their enzymatic activities, the mitochondrial membrane potential, and the production of ATP and ROS. Our model is discussed through two distinct human pathologies, acute inflammation as seen in sepsis, where phosphorylation leads to strong COX inhibition followed by energy depletion, and ischemia/reperfusion injury, where hyperactive ETC complexes generate pathologically high mitochondrial membrane potentials, leading to excessive ROS production. Although operating at opposite poles of the ETC activity spectrum, both conditions can lead to cell death through energy deprivation or ROS-triggered apoptosis.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures


References
-
- Rich P. Chemiosmotic coupling: The cost of living. Nature. 2003;421:583. - PubMed
-
- Morriss GM, New DA. Effect of oxygen concentration on morphogenesis of cranial neural folds and neural crest in cultured rat embryos. J Embryol Exp Morphol. 1979;54:17–35. - PubMed
-
- Warburg O, Posener K, Negelein E. Über den Stoffwechsel der Carcinomzelle. Biochem Z. 1924;152:309–344.
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Lardy HA, Wellman H. Oxidative phosphorylations; role of inorganic phosphate and acceptor systems in control of metabolic rates. J Biol Chem. 1952;195:215–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

