α1-adrenergic receptors facilitate inhibitory neurotransmission to cardiac vagal neurons in the nucleus ambiguus
- PMID: 21771639
- PMCID: PMC3171606
- DOI: 10.1016/j.neuroscience.2011.07.024
α1-adrenergic receptors facilitate inhibitory neurotransmission to cardiac vagal neurons in the nucleus ambiguus
Abstract
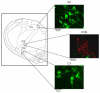
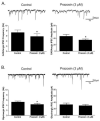
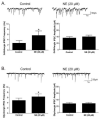
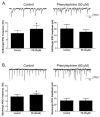
The cholinergic cardiac vagal neurons (CVNs), located in the nucleus ambiguus, are the origin of cardioinhibitory parasympathetic activity. Catecholaminergic neurons in nearby regions of the brainstem, including the C1 and C2 cell groups, are thought to play a key role in both arousing from sleep and maintaining wakefulness. Because norepinephrine (NE) could play an important role in influencing the activity of CVNs, particularly in response to sleeping/waking and arousal states, the present study investigated the contribution of α(1)-adrenergic receptor activation to augment inhibitory and/or blunt excitatory neurotransmission to CVNs. To test the effects of α(1)-adrenergic receptor activation, CVNs were labeled in rats by retrograde tracing and synaptic events were recorded by whole cell voltage clamp techniques in vitro. Prazosin, an inverse agonist of α(1)-adrenergic receptor, significantly decreased the frequency of both GABAergic and glycinergic neurotransmission to CVNs. Activation of α(1)-adrenergic receptors by the α(1)-adrenergic receptor agonists NE or phenylephrine (PE) both significantly increased GABAergic and glycinergic inhibitory event frequency. This effect was prevented by the sodium channel blocker tetrodotoxin (TTX). Activation of α(1)-adrenergic receptors did not alter glutamatergic neurotransmission to CVNs. This study indicates that α(1)-adrenergic receptor activation in the brainstem can facilitate inhibitory GABAergic and glycinergic neurotransmission so as to reduce CVN activity; this synaptic modulation may play a role in the tachycardia seen during NE-dependent behavioral arousal.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- Baba H, Shimoji K, Yoshimura M. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 1): effects on axon terminals of GABAergic and glycinergic neurons. Anesthesiology. 2000;92:473–484. - PubMed
-
- Bhuiyan ME, Waki H, Gouraud SS, Takagishi M, Cui H, Yamazaki T, Kohsaka A, Maeda M. Complex cardiovascular actions of alpha-adrenergic receptors expressed in the nucleus tractus solitarii of rats. Exp Physiol. 2009;94:773–784. - PubMed
-
- Day HE, Campeau S, Watson SJ, Jr., Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

