Specific enzyme complex of beta-1,4-galactosyltransferase-II and glucuronyltransferase-P facilitates biosynthesis of N-linked human natural killer-1 (HNK-1) carbohydrate
- PMID: 21771787
- PMCID: PMC3173110
- DOI: 10.1074/jbc.M111.233353
Specific enzyme complex of beta-1,4-galactosyltransferase-II and glucuronyltransferase-P facilitates biosynthesis of N-linked human natural killer-1 (HNK-1) carbohydrate
Abstract
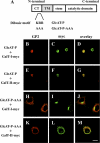
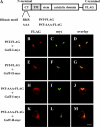
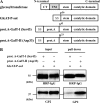
Human natural killer-1 (HNK-1) carbohydrate is highly expressed in the nervous system and is involved in synaptic plasticity and dendritic spine maturation. This unique carbohydrate, consisting of a sulfated trisaccharide (HSO(3)-3GlcAβ1-3Galβ1-4GlcNAc-), is biosynthesized by the successive actions of β-1,4-galactosyltransferase (β4GalT), glucuronyltransferase (GlcAT-P and GlcAT-S), and sulfotransferase (HNK-1ST). A previous study showed that mice lacking β4GalT-II, one of seven β4GalTs, exhibited a dramatic loss of HNK-1 expression in the brain, although β4GalT-I-deficient mice did not. Here, we investigated the underlying molecular mechanism of the regulation of HNK-1 expression. First, focusing on a major HNK-1 carrier, neural cell adhesion molecule, we found that reduced expression of an N-linked HNK-1 carbohydrate caused by a deficiency of β4GalT-II is not likely due to a general loss of the β1,4-galactose residue as an acceptor for GlcAT-P. Instead, we demonstrated by co-immunoprecipitation and endoplasmic reticulum-retention analyses using Neuro2a (N2a) cells that β4GalT-II physically and specifically associates with GlcAT-P. In addition, we revealed by pulldown assay that Golgi luminal domains of β4GalT-II and GlcAT-P are sufficient for the complex to form. With an in vitro assay system, we produced the evidence that the kinetic efficiency k(cat)/K(m) of GlcAT-P in the presence of β4GalT-II was increased about 2.5-fold compared with that in the absence of β4GalT-II. Finally, we showed that co-expression of β4GalT-II and GlcAT-P increased HNK-1 expression on various glycoproteins in N2a cells, including neural cell adhesion molecule. These results indicate that the specific enzyme complex of β4GalT-II with GlcAT-P plays an important role in the biosynthesis of HNK-1 carbohydrate.
Figures





References
-
- Ohtsubo K., Marth J. D. (2006) Cell 126, 855–867 - PubMed
-
- Lowe J. B., Marth J. D. (2003) Annu. Rev. Biochem. 72, 643–691 - PubMed
-
- Sugimoto I., Futakawa S., Oka R., Ogawa K., Marth J. D., Miyoshi E., Taniguchi N., Hashimoto Y., Kitazume S. (2007) J. Biol. Chem. 282, 34896–34903 - PubMed
-
- Seko A. (2006) Trends Glycosci. Glycotechnol. 18, 209–230
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

